"V体育平台登录" Evidence that CXCL16 is a potent mediator of angiogenesis and is involved in endothelial progenitor cell chemotaxis : studies in mice with K/BxN serum-induced arthritis
- PMID: 23633118
- PMCID: PMC3701743
- DOI: "V体育官网" 10.1002/art.37981
Evidence that CXCL16 is a potent mediator of angiogenesis and is involved in endothelial progenitor cell chemotaxis : studies in mice with K/BxN serum-induced arthritis
"VSports注册入口" Abstract
Objective: To examine the possibility that CXCL16 recruits endothelial cells (ECs) to developing neovasculature in rheumatoid arthritis (RA) synovium VSports手机版. .
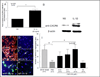
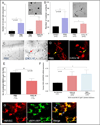
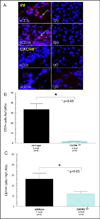
Methods: We utilized the RA synovial tissue SCID mouse chimera system to examine human microvascular EC (HMVEC) and human endothelial progenitor cell (EPC) recruitment into engrafted human synovium that was injected intragraft with CXCL16-immunodepleted RA synovial fluid (SF). CXCR6-deficient and wild-type (WT) C57BL/6 mice were primed to develop K/BxN serum-induced arthritis and evaluated for angiogenesis V体育安卓版. HMVECs and EPCs from human cord blood were also examined for CXCR6 expression, by immunofluorescence and assessment of CXCL16 signaling activity. .
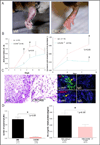
Results: CXCR6 was prominently expressed on human EPCs and HMVECs, and its expression on HMVECs could be up-regulated by interleukin-1β. SCID mice injected with CXCL16-depleted RA SF exhibited a significant reduction in EPC recruitment. In experiments using the K/BxN serum-induced inflammatory arthritis model, CXCR6(-/-) mice showed profound reductions in hemoglobin levels, which correlated with reductions in monocyte and T cell recruitment to arthritic joint tissue compared to that observed in WT mice. Additionally, HMVECs and EPCs responded to CXCL16 stimulation, but exhibited unique signal transduction pathways and homing properties. V体育ios版.
Conclusion: These results indicate that CXCL16 and its receptor CXCR6 may be a central ligand/receptor pair that is closely associated with EPC recruitment and blood vessel formation in the RA joint. VSports最新版本.
Copyright © 2013 by the American College of Rheumatology. V体育平台登录.
Conflict of interest statement
Conflicts of interest: none
Figures


References
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. - PubMed
-
- Corrigall VM, Panayi GS. Autoantigens and immune pathways in rheumatoid arthritis. Crit Rev Immunol. 2002;22(4):281–293. - "VSports在线直播" PubMed
-
- Paleolog EM. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):S81–S90. - PMC (V体育ios版) - PubMed
-
- Folkman J, Haudenschild C. Angiogenesis by capillary endothelial cells in culture. Trans Ophthalmol Soc U K. 1980;100(3):346–353. - PubMed
-
- Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58(2):390–398. - PubMed
Publication types
- Actions (VSports在线直播)
MeSH terms
- V体育2025版 - Actions
- Actions (V体育官网入口)
- V体育ios版 - Actions
- VSports最新版本 - Actions
- VSports - Actions
- Actions (V体育平台登录)
- "VSports注册入口" Actions
- Actions (VSports app下载)
- VSports最新版本 - Actions
- "V体育ios版" Actions
- "V体育平台登录" Actions
Substances
- VSports在线直播 - Actions
- Actions (V体育官网入口)
- "V体育2025版" Actions
- "V体育ios版" Actions
- "V体育安卓版" Actions
- "VSports最新版本" Actions
- V体育平台登录 - Actions
Grants and funding
- "VSports app下载" R01 CA172048/CA/NCI NIH HHS/United States
- "VSports注册入口" R01-CA-122031/CA/NCI NIH HHS/United States
- R01 HL058695/HL/NHLBI NIH HHS/United States (V体育官网入口)
- V体育官网 - R01 CA160216/CA/NCI NIH HHS/United States
- R01 CA122031/CA/NCI NIH HHS/United States
- P30 AR048310/AR/NIAMS NIH HHS/United States
- "V体育2025版" P30-AR-048310/AR/NIAMS NIH HHS/United States
- R01 AI040987/AI/NIAID NIH HHS/United States
- HL-58694/HL/NHLBI NIH HHS/United States (V体育官网)
- AR-48267/AR/NIAMS NIH HHS/United States
- R01 AR048267/AR/NIAMS NIH HHS/United States
- AI-40987/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

