Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment
- PMID: 23624405
- PMCID: PMC4156024
- DOI: 10.1038/ncb2730
Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment
"VSports" Erratum in
- Nat Cell Biol. 2013 Aug;15(8):1016
V体育2025版 - Abstract
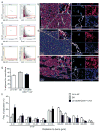
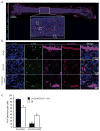
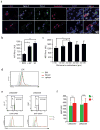
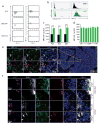
The existence of a haematopoietic stem cell niche as a spatially confined regulatory entity relies on the notion that haematopoietic stem and progenitor cells (HSPCs) are strategically positioned in unique bone marrow microenvironments with defined anatomical and functional features. Here, we employ a powerful imaging cytometry platform to perform a comprehensive quantitative analysis of HSPC distribution in bone marrow cavities of femoral bones. We find that HSPCs preferentially localize in endosteal zones, where most closely interact with sinusoidal and non-sinusoidal bone marrow microvessels, which form a distinctive circulatory system VSports手机版. In situ tissue analysis reveals that HSPCs exhibit a hypoxic profile, defined by strong retention of pimonidazole and expression of HIF-1α, regardless of localization throughout the bone marrow, adjacency to vascular structures or cell-cycle status. These studies argue that the characteristic hypoxic state of HSPCs is not solely the result of a minimally oxygenated niche but may be partially regulated by cell-specific mechanisms. .
"VSports在线直播" Conflict of interest statement
The authors declare no competing financial interests.
V体育ios版 - Figures



References
Publication types
MeSH terms
- "V体育2025版" Actions
- "V体育平台登录" Actions
- "V体育2025版" Actions
- "VSports最新版本" Actions
- VSports最新版本 - Actions
- "V体育ios版" Actions
- V体育ios版 - Actions
- Actions (V体育2025版)
- VSports app下载 - Actions
- "V体育安卓版" Actions
V体育官网入口 - Substances
- V体育官网 - Actions
- VSports注册入口 - Actions
- Actions (VSports最新版本)
"V体育2025版" Grants and funding
LinkOut - more resources (V体育ios版)
Full Text Sources
Other Literature Sources
Medical
"V体育ios版" Molecular Biology Databases

