"VSports注册入口" Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells
- PMID: 23536763
- PMCID: PMC3594239
- DOI: 10.1371/journal.pone.0057188
Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells
Erratum in
- PLoS One. 2013;8(12). doi:10.1371/annotation/aed5b555-b826-4591-8aa6-284ad888627d
Abstract
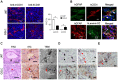
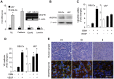
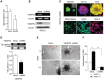
Human glioblastomas (GBM) are thought to be initiated by glioma stem-like cells (GSLCs). GSLCs also participate in tumor neovascularization by transdifferentiating into vascular endothelial cells. Here, we report a critical role of GSLCs in the formation of vasculogenic mimicry (VM), which defines channels lined by tumor cells to supply nutrients to early growing tumors and tumor initiation. GSLCs preferentially expressed vascular endothelial growth factor receptor-2 (VEGFR-2) that upon activation by VEGF, mediated chemotaxis, tubule formation and increased expression of critical VM markers by GSLCs. Knockdown of VEGFR-2 in GSLCs by shRNA markedly reduced their capacity of self-renewal, forming tubules, initiating xenograft tumors, promoting vascularization and the establishment of VM VSports手机版. Our study demonstrates VEGFR-2 as an essential molecule to sustain the "stemness" of GSLCs, their capacity to initiate tumor vasculature, and direct initiation of tumor. .
Conflict of interest statement
Figures



References
-
- Ausprunk DH, Folkman J (1997) Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 14: 53–65. - PubMed
-
- Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, et al. (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiform. J Clin Oncol 25: 4722–4729. - PubMed
-
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, et al. (1999) Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284: 1994–1998. - PubMed
V体育官网入口 - Publication types
MeSH terms
- Actions (V体育2025版)
- V体育ios版 - Actions
- "V体育官网入口" Actions
- Actions (VSports)
- V体育平台登录 - Actions
- VSports在线直播 - Actions
- Actions (VSports)
- V体育官网入口 - Actions
- Actions (V体育2025版)
Substances (VSports)
- V体育2025版 - Actions
- "VSports最新版本" Actions
- "VSports" Actions
Grants and funding (VSports注册入口)
"VSports app下载" LinkOut - more resources
Full Text Sources
VSports - Other Literature Sources
Molecular Biology Databases

