"V体育ios版" Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen-independent prostate cancer cells by downregulating macrophage inhibitory cytokine-1
- PMID: 23449353
- PMCID: PMC3619053
- DOI: VSports最新版本 - 10.1038/bjc.2012.484
V体育ios版 - Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen-independent prostate cancer cells by downregulating macrophage inhibitory cytokine-1
Abstract
Background: Overexpression of macrophage inhibitory cytokine-1 (MIC-1) frequently occurs during the progression of prostate cancer (PC) to androgen-independent (AI) and metastatic disease states and is associated with a poor outcome of patients VSports手机版. .
Methods: The gain- and loss-of-function analyses of MIC-1 were performed to establish its implications for aggressive and chemoresistant phenotypes of metastatic and AI PC cells and the benefit of its downregulation for reversing docetaxel resistance. V体育安卓版.
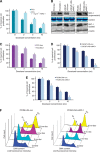
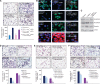
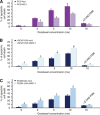
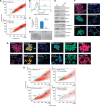
Results: The results have indicated that an enhanced level of secreted MIC-1 protein in PC3 cells is associated with their acquisition of epithelial-mesenchymal transition features and higher invasive capacity and docetaxel resistance. Importantly, the downregulation of MIC-1 in LNCaP-LN3 and PC3M-LN4 cells significantly decreased their invasive capacity and promoted the antiproliferative, anti-invasive and mitochrondrial- and caspase-dependent apoptotic effects induced by docetaxel V体育ios版. The downregulation of MIC-1 in PC3M-LN4 cells was also effective in promoting the cytotoxic effects induced by docetaxel on the side population (SP) endowed with stem cell-like properties and the non-SP cell fraction from PC3M-LN4 cells. .
Conclusion: These data suggest that the downregulation of MIC-1 may constitute a potential therapeutic strategy for improving the efficacy of current docetaxel-based chemotherapies, eradicating the total mass of PC cells and thereby preventing disease relapse and the death of PC patients. VSports最新版本.
"VSports app下载" Figures


References
-
- Bauskin AR, Brown DA, Junankar S, Rasiah KK, Eggleton S, Hunter M, Liu T, Smith D, Kuffner T, Pankhurst GJ, Johnen H, Russell PJ, Barret W, Stricker PD, Grygiel JJ, Kench JG, Henshall SM, Sutherland RL, Breit SN. The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res. 2005;65:2330–2336. - PubMed
-
- Birnie R, Bryce SD, Roome C, Dussupt V, Droop A, Lang SH, Berry PA, Hyde CF, Lewis JL, Stower MJ, Maitland NJ, Collins AT. Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 2008;9:R83. - PMC - PubMed
-
- Boyle GM, Pedley J, Martyn AC, Banducci KJ, Strutton GM, Brown DA, Breit SN, Parsons PG. Macrophage inhibitory cytokine-1 is overexpressed in malignant melanoma and is associated with tumorigenicity. J Invest Dermatol. 2009;129:383–391. - VSports在线直播 - PubMed
-
- Brown DA, Lindmark F, Stattin P, Balter K, Adami HO, Zheng SL, Xu J, Isaacs WB, Gronberg H, Breit SN, Wiklund FE. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin Cancer Res. 2009;15:6658–6664. - PMC (VSports注册入口) - PubMed
Publication types
- "V体育安卓版" Actions
MeSH terms
- Actions (VSports在线直播)
- V体育ios版 - Actions
- V体育ios版 - Actions
- V体育安卓版 - Actions
- Actions (VSports在线直播)
- "VSports app下载" Actions
- "VSports在线直播" Actions
- Actions (V体育官网入口)
- Actions (V体育2025版)
- V体育官网入口 - Actions
V体育2025版 - Substances
- Actions (V体育安卓版)
- Actions (VSports app下载)
- "VSports app下载" Actions
"VSports最新版本" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育官网 - Medical

