miR-210 is a target of hypoxia-inducible factors 1 and 2 in renal cancer, regulates ISCU and correlates with good prognosis
- PMID: 23449350
- PMCID: VSports最新版本 - PMC3619073
- DOI: V体育ios版 - 10.1038/bjc.2013.56
miR-210 is a target of hypoxia-inducible factors 1 and 2 in renal cancer, regulates ISCU and correlates with good prognosis
Abstract (VSports app下载)
Background: Clear cell renal cancer frequently harbours von Hippel-Lindau (VHL) gene mutations, leading to stabilisation of the hypoxia-inducible factors (HIFs) and expression of their target genes. We investigated HIF-1 and HIF-2 in the regulation of microRNA-210 (miR-210), and its clinical relevance in renal tumours. VSports手机版.
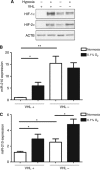
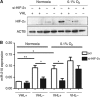
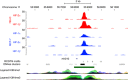
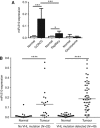
Methods: RCC4 and 786-O renal cancer cell lines transfected with either an empty vector or functional VHL and incubated in normoxia or hypoxia were examined for miR-210 expression. Hypoxia-inducible factor siRNAs were used to examine their regulation of miR-210. Seventy-one clear cell renal tumours were sequenced for VHL mutations. Expression of miR-210, VHL, CA9, ISCU and Ki-67 were determined by immunohistochemistry and qRT-PCR V体育安卓版. .
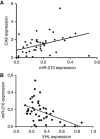
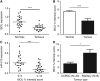
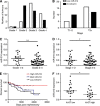
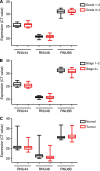
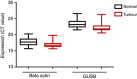
Results: In addition to HIF-1 regulating miR-210 in renal cancer, HIF-2 can regulate this microRNA in the absence of HIF-1. MicroRNA-210 is upregulated in renal cancer compared with normal renal cortex tissue. MicroRNA-210 correlates negatively with its gene target ISCU at the protein and mRNA level V体育ios版. MicroRNA-210 correlated with positive outcome variables and negatively with Ki-67. .
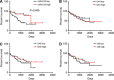
Conclusion: We provide further evidence of miR-210 activity in vivo, and show that high miR-210 expression is associated with better clinico-pathological prognostic factors VSports最新版本. .
Figures
References
-
- Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, Stanbridge E, Lerman MI, Palotie A, Figlin RA, Belldegrun AS. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. - PubMed
-
- Bui MH, Visapaa H, Seligson D, Kim H, Han KR, Huang Y, Horvath S, Stanbridge EJ, Palotie A, Figlin RA, Belldegrun AS. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171:2461–2466. - "VSports app下载" PubMed
-
- Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. - PubMed (VSports最新版本)
Publication types
MeSH terms
- V体育ios版 - Actions
- Actions (VSports最新版本)
- V体育2025版 - Actions
- "V体育平台登录" Actions
- Actions (VSports app下载)
- "V体育ios版" Actions
- V体育官网 - Actions
- Actions (V体育官网)
- Actions (VSports最新版本)
- VSports - Actions
- Actions (VSports在线直播)
Substances
- VSports app下载 - Actions
- Actions (VSports)
- "VSports注册入口" Actions
- "VSports手机版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

