Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements
- PMID: 23374342
- PMCID: PMC3629564 (VSports手机版)
- DOI: 10.1016/j.cell.2012.12.023
Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements
Abstract
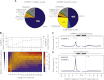
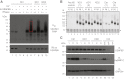
There are ~650,000 Alu elements in transcribed regions of the human genome. These elements contain cryptic splice sites, so they are in constant danger of aberrant incorporation into mature transcripts. Despite posing a major threat to transcriptome integrity, little is known about the molecular mechanisms preventing their inclusion. Here, we present a mechanism for protecting the human transcriptome from the aberrant exonization of transposable elements. Quantitative iCLIP data show that the RNA-binding protein hnRNP C competes with the splicing factor U2AF65 at many genuine and cryptic splice sites. Loss of hnRNP C leads to formation of previously suppressed Alu exons, which severely disrupt transcript function VSports手机版. Minigene experiments explain disease-associated mutations in Alu elements that hamper hnRNP C binding. Thus, by preventing U2AF65 binding to Alu elements, hnRNP C plays a critical role as a genome-wide sentinel protecting the transcriptome. The findings have important implications for human evolution and disease. .
Copyright © 2013 Elsevier Inc. All rights reserved V体育安卓版. .
Figures











Comment in
-
Alternative splicing: Regulating Alu element 'exonization'.Nat Rev Genet. 2013 Mar;14(3):152-3. doi: 10.1038/nrg3428. Epub 2013 Feb 5. Nat Rev Genet. 2013. PMID: 23381119 No abstract available.
References
-
- Beyer A.L., Christensen M.E., Walker B.W., LeStourgeon W.M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977;11:127–138. - PubMed
-
- Buratti E., Chivers M., Královicová J., Romano M., Baralle M., Krainer A.R., Vorechovsky I. Aberrant 5′ splice sites in human disease genes: mutation pattern, nucleotide structure and comparison of computational tools that predict their utilization. Nucleic Acids Res. 2007;35:4250–4263. - PMC - PubMed
-
- Caras I.W., Davitz M.A., Rhee L., Weddell G., Martin D.W., Jr., Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987;325:545–549. - VSports最新版本 - PubMed
Supplemental References
-
- Löytynoja A., Goldman N. Phylogeny-aware gap placement prevents errors in sequence alignment and evolutionary analysis. Science. 2008;320:1632–1635. - PubMed
-
- Smit, A., Hubley, R., and Green, P. (1996–2010). RepeatMasker Open-3.0. http://wwwrepeatmaskerorg.
Publication types
MeSH terms
- "VSports" Actions
- VSports在线直播 - Actions
- VSports app下载 - Actions
- Actions (VSports app下载)
- Actions (VSports在线直播)
- Actions (VSports注册入口)
- "V体育安卓版" Actions
- VSports - Actions
- V体育2025版 - Actions
"VSports最新版本" Substances
- "VSports最新版本" Actions
- "V体育2025版" Actions
- "V体育官网入口" Actions
Grants and funding
LinkOut - more resources
"V体育官网" Full Text Sources
Other Literature Sources
Molecular Biology Databases

