Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE): a randomized controlled trial protocol
- PMID: 23311856
- PMCID: PMC3547701
- DOI: 10.1186/1471-2377-13-5
Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE): a randomized controlled trial protocol
Abstract
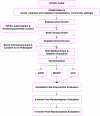
Background: Residual disability after stroke is substantial; 65% of patients at 6 months are unable to incorporate the impaired upper extremity into daily activities. Task-oriented training programs are rapidly being adopted into clinical practice. In the absence of any consensus on the essential elements or dose of task-specific training, an urgent need exists for a well-designed trial to determine the effectiveness of a specific multidimensional task-based program governed by a comprehensive set of evidence-based principles VSports手机版. The Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE) Stroke Initiative is a parallel group, three-arm, single blind, superiority randomized controlled trial of a theoretically-defensible, upper extremity rehabilitation program provided in the outpatient setting. The primary objective of ICARE is to determine if there is a greater improvement in arm and hand recovery one year after randomization in participants receiving a structured training program termed Accelerated Skill Acquisition Program (ASAP), compared to participants receiving usual and customary therapy of an equivalent dose (DEUCC). Two secondary objectives are to compare ASAP to a true (active monitoring only) usual and customary (UCC) therapy group and to compare DEUCC and UCC. .
Methods/design: Following baseline assessment, participants are randomized by site, stratified for stroke duration and motor severity. 360 adults will be randomized, 14 to 106 days following ischemic or hemorrhagic stroke onset, with mild to moderate upper extremity impairment, recruited at sites in Atlanta, Los Angeles and Washington, D. C. The Wolf Motor Function Test (WMFT) time score is the primary outcome at 1 year post-randomization. The Stroke Impact Scale (SIS) hand domain is a secondary outcome measure. The design includes concealed allocation during recruitment, screening and baseline, blinded outcome assessment and intention to treat analyses. Our primary hypothesis is that the improvement in log-transformed WMFT time will be greater for the ASAP than the DEUCC group. This pre-planned hypothesis will be tested at a significance level of 0 V体育安卓版. 05. .
Discussion: ICARE will test whether ASAP is superior to the same number of hours of usual therapy. Pre-specified secondary analyses will test whether 30 hours of usual therapy is superior to current usual and customary therapy not controlled for dose. V体育ios版.
Trial registration: www VSports最新版本. ClinicalTrials. gov Identifier: NCT00871715 .
Figures

"VSports" References
-
- Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83(8):1035–1042. doi: 10.1053/apmr.2002.33984. - "V体育平台登录" DOI - PubMed
-
- Gresham GE, Duncan PW, Stason WB, Adams HP, Jr, Adelman AM, Alexander DN, Bishop DS, Diller L, Donaldson NE, Granger CV, Post-Stroke Rehabilitation. Rockville, MD: U.S. Department of Health and Human Services; 1995.
-
- Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the stroke impact scale. Stroke. 2002;33(7):1840–1844. doi: 10.1161/01.STR.0000019289.15440.F2. - V体育平台登录 - DOI - PubMed
V体育ios版 - Publication types
- V体育2025版 - Actions
V体育ios版 - MeSH terms
- "VSports手机版" Actions
- "V体育ios版" Actions
- V体育官网 - Actions
- "V体育官网" Actions
- "VSports app下载" Actions
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- Actions (V体育官网)
- "VSports手机版" Actions
VSports - Associated data
- Actions (VSports注册入口)
Grants and funding
VSports手机版 - LinkOut - more resources
"VSports最新版本" Full Text Sources
Other Literature Sources
Medical

