Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases (V体育官网)
- PMID: 23249624
- PMCID: PMC3583506
- DOI: 10.1124/jpet.112.201475
Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases
Abstract (V体育平台登录)
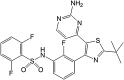
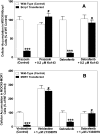
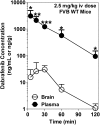
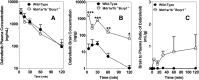
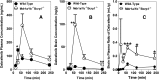
Brain metastases are a common cause of death in stage IV metastatic melanoma. Dabrafenib is a BRAF (gene encoding serine/threonine-protein kinase B-Raf) inhibitor that has been developed to selectively target the valine 600 to glutamic acid substitution (BRAF(V600E)), which is commonly found in metastatic melanoma. Clinical trials with dabrafenib have shown encouraging results; however, the central nervous system distribution of dabrafenib remains unknown. Thus, the objective of the current study was to evaluate the brain distribution of dabrafenib in mice, and to see whether active efflux by P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) restricts its delivery across the blood-brain barrier (BBB). In vitro accumulation studies conducted in Madin-Darby canine kidney II cells indicate that dabrafenib is an avid substrate for both P-gp and BCRP. Directional flux studies revealed greater transport in the basolateral to apical direction with corrected efflux ratios greater than 2 for both P-gp and Bcrp1 transfected cell lines VSports手机版. In vivo, the ratio of area under the concentration-time curve (AUC)(brain) to AUC(plasma) (K(p)) of dabrafenib after an i. v. dose (2. 5 mg/kg) was 0. 023, which increased by 18-fold in Mdr1 a/b(-/-)Bcrp1(-/-) mice to 0. 42. Dabrafenib plasma exposure was ∼2-fold greater in Mdr1 a/b(-/-)Bcrp1(-/-) mice as compared with wild-type with an oral dose (25 mg/kg); however, the brain distribution was increased by ~10-fold with a resulting K(p) of 0. 25. Further, compared with vemurafenib, another BRAF(V600E) inhibitor, dabrafenib showed greater brain penetration with a similar dose. In conclusion, the dabrafenib brain distribution is limited in an intact BBB model, and the data presented herein may have clinical implications in the prevention and treatment of melanoma brain metastases. .
"V体育2025版" Figures


References
-
- Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. (2010) Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther 334:147–155 - "V体育平台登录" PMC - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17:2105–2116 - PubMed
-
- Bailer AJ. (1988) Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm 16:303–309 - "V体育官网" PubMed
Publication types
- Actions (V体育安卓版)
MeSH terms
- Actions (V体育安卓版)
- V体育平台登录 - Actions
- "V体育官网入口" Actions
- V体育ios版 - Actions
- "VSports手机版" Actions
- V体育ios版 - Actions
- "V体育ios版" Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
- Actions (V体育2025版)
- "V体育官网" Actions
- Actions (V体育2025版)
- "VSports" Actions
- V体育官网 - Actions
Substances (V体育ios版)
- V体育官网入口 - Actions
- VSports在线直播 - Actions
- "VSports" Actions
Grants and funding
LinkOut - more resources
"V体育平台登录" Full Text Sources
Other Literature Sources
Medical
V体育安卓版 - Research Materials
Miscellaneous

