"V体育2025版" Hypomethylating therapy in an aggressive stroma-rich model of pancreatic carcinoma
- PMID: 23204224
- PMCID: PMC3548986
- DOI: 10.1158/0008-5472.CAN-12-1880
V体育安卓版 - Hypomethylating therapy in an aggressive stroma-rich model of pancreatic carcinoma
Abstract
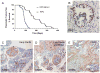
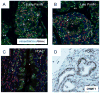
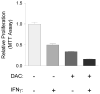
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy that resists current treatments. To test epigenetic therapy against this cancer, we used the DNA demethylating drug 5-aza-2'-deoxycytidine (DAC) in an aggressive mouse model of stromal rich PDAC (KPC-Brca1 mice). In untreated tumors, we found globally decreased 5-methyl-cytosine (5-mC) in malignant epithelial cells and in cancer-associated myofibroblasts (CAF), along with increased amounts of 5-hydroxymethyl-cytosine (5-HmC) in CAFs, in progression from pancreatic intraepithelial neoplasia to PDAC. DAC further reduced DNA methylation and slowed PDAC progression, markedly extending survival in an early-treatment protocol and significantly though transiently inhibiting tumor growth when initiated later, without adverse side effects. Escaping tumors contained areas of sarcomatoid transformation with disappearance of CAFs. Mixing-allografting experiments and proliferation indices showed that DAC efficacy was due to inhibition of both the malignant epithelial cells and the CAFs. Expression profiling and immunohistochemistry highlighted DAC induction of STAT1 in the tumors, and DAC plus IFN-γ produced an additive antiproliferative effect on PDAC cells VSports手机版. DAC induced strong expression of the testis antigen deleted in azoospermia-like (DAZL) in CAFs. These data show that DAC is effective against PDAC in vivo and provide a rationale for future studies combining hypomethylating agents with cytokines and immunotherapy. .
Conflict of interest statement
Conflict of Interest: There are no conflicts to declare.
Figures



References
-
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. - PubMed
-
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. - PubMed
-
- Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–2090. - PubMed
-
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. - PubMed
-
- Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. - VSports手机版 - PubMed
"VSports在线直播" Publication types
MeSH terms
- Actions (VSports)
- Actions (VSports app下载)
- Actions (V体育官网)
- VSports最新版本 - Actions
- Actions (V体育2025版)
- V体育2025版 - Actions
Substances
Grants and funding
LinkOut - more resources
Full Text Sources (V体育2025版)
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

