VSports注册入口 - WNT10A plays an oncogenic role in renal cell carcinoma by activating WNT/β-catenin pathway
- PMID: 23094073
- PMCID: PMC3477117
- DOI: 10.1371/journal.pone.0047649 (VSports手机版)
WNT10A plays an oncogenic role in renal cell carcinoma by activating WNT/β-catenin pathway
Abstract (VSports在线直播)
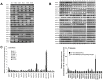
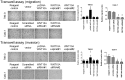
Renal cell carcinoma (RCC) is a malignancy with poor prognosis VSports手机版. WNT/β-catenin signaling dysregulation, especially β-catenin overactivation and WNT antagonist silencing, is associated with RCC carcinogenesis and progression. However, the role of WNT ligands in RCC has not yet been determined. We screened 19 WNT ligands from normal kidney and RCC cell lines and tissues and found that WNT10A was significantly increased in RCC cell lines and tissues as compared to that in normal controls. The clinical significance of increase in WNT10A was evaluated by performing an immunohistochemical association study in a 19-year follow-up cohort comprising 284 RCC and 267 benign renal disease (BRD) patients. The results of this study showed that WNT10A was dramatically upregulated in RCC tissues as compared to that in BRD tissues. This result suggests that WNT10A, nuclear β-catenin, and nuclear cyclin D1 act as independent risk factors for RCC carcinogenesis and progression, with accumulative risk effects. Molecular validation of cell line models with gain- or loss-of-function designs showed that forced WNT10A expression induced RCC cell proliferation and aggressiveness, including higher chemoresistance, cell migration, invasiveness, and cell transformation, due to the activation of β-catenin-dependent signaling. Conversely, WNT10A siRNA knockdown decreased cell proliferation and aggressiveness of RCC cells. In conclusion, we showed that WNT10A acts as an autocrine oncogene both in RCC carcinogenesis and progression by activating WNT/β-catenin signaling. .
Conflict of interest statement
"V体育平台登录" Figures






VSports手机版 - References
-
- Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, et al. (2011) The epidemiology of renal cell carcinoma. Eur Urol 60: 615–621. - PubMed
-
- Bureau of Health Promotion, Department of Health, ROC (Taiwan) website. Available: http://www.bhp.doh.gov.tw/BHPnet/English/Class.aspx?Sub=statistics&No=20.... Accessed 2012 Sep 25.
-
- Schedl A (2007) Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802. - PubMed (V体育安卓版)
Publication types
- VSports在线直播 - Actions
MeSH terms
- VSports - Actions
- "VSports手机版" Actions
- Actions (VSports app下载)
- Actions (VSports手机版)
- "V体育ios版" Actions
- "V体育2025版" Actions
- "VSports" Actions
- Actions (VSports)
- "V体育ios版" Actions
- Actions (VSports注册入口)
- Actions (VSports在线直播)
- "V体育官网" Actions
- VSports手机版 - Actions
- "VSports手机版" Actions
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- "VSports app下载" Actions
V体育2025版 - Substances
- "VSports最新版本" Actions
- Actions (VSports手机版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
"VSports在线直播" Research Materials

