Rad51 is an accessory factor for Dmc1-mediated joint molecule formation during meiosis
- PMID: 22955832
- PMCID: VSports - PMC4056682
- DOI: "V体育安卓版" 10.1126/science.1219379
Rad51 is an accessory factor for Dmc1-mediated joint molecule formation during meiosis
Abstract
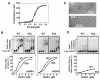
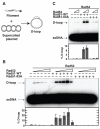
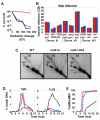
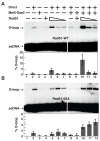
Meiotic recombination in budding yeast requires two RecA-related proteins, Rad51 and Dmc1, both of which form filaments on DNA capable of directing homology search and catalyzing formation of homologous joint molecules (JMs) and strand exchange VSports手机版. With use of a separation-of-function mutant form of Rad51 that retains filament-forming but not JM-forming activity, we show that the JM activity of Rad51 is fully dispensable for meiotic recombination. The corresponding mutation in Dmc1 causes a profound recombination defect, demonstrating Dmc1's JM activity alone is responsible for meiotic recombination. We further provide biochemical evidence that Rad51 acts with Mei5-Sae3 as a Dmc1 accessory factor. Thus, Rad51 is a multifunctional protein that catalyzes recombination directly in mitosis and indirectly, via Dmc1, during meiosis. .
"VSports app下载" Figures
References
-
- Bishop DK, Park D, Xu L, Kleckner N. Cell. 1992;69:439. - PubMed
-
- Shinohara A, Ogawa H, Ogawa T. Cell. 1992;69:457. - PubMed
-
- Schwacha A, Kleckner N. Cell. 1997 Sep 19;90:1123. - PubMed
-
- Sheridan S, Bishop DK. Genes Dev. 2006 Jul 1;20:1685. - PubMed (V体育2025版)
Publication types
"V体育平台登录" MeSH terms
- Actions (V体育平台登录)
- Actions (VSports最新版本)
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- "VSports app下载" Actions
- Actions (VSports注册入口)
- "VSports app下载" Actions
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- "V体育平台登录" Actions
- Actions (V体育平台登录)
Substances
- V体育2025版 - Actions
- V体育官网 - Actions
- "VSports" Actions
- "VSports手机版" Actions
- "VSports注册入口" Actions
- V体育平台登录 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources (VSports手机版)
Other Literature Sources
Molecular Biology Databases (V体育平台登录)
Research Materials (VSports app下载)

