VSports最新版本 - IKK-ε coordinates invasion and metastasis of ovarian cancer
- PMID: 22942254
- PMCID: PMC3488159
- DOI: 10.1158/0008-5472.CAN-11-3993
IKK-ε coordinates invasion and metastasis of ovarian cancer
Abstract
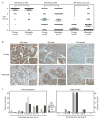
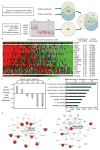
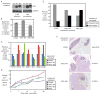
Inhibitor of IκB kinases (IKK) are key regulators of NF-κB signaling. Three IKK isoforms-α, β, and ε-have been linked to oncogenesis, yet the precise components of NF-κB signaling in ovarian cancer have not yet been dissected. We surveyed 120 ovarian cancer specimens for IKK-ε expression. Notably, cytoplasmic expression was elevated in metastatic lesions relative to primary tumors (P = 0. 03) VSports手机版. Therefore, we hypothesized that IKK-ε drives ovarian cancer metastasis. IKK-ε was identified previously as a breast cancer oncogene and was associated with poor clinical outcome in ovarian cancer. We now define an ovarian cancer-specific IKK-ε-regulated gene expression signature using stably expressed short hairpin RNA targeting IKK-ε. Pathway analysis of the signature indicated that IKK-ε regulates expression of genes involved in cell motility and inflammation. We further showed that IKK-ε depletion in metastatic ovarian cancer cell lines decreased growth, adhesion, and invasion. Consistently, human xenografts depleted of IKK-ε in mice showed decreased aggressiveness, whereas overexpression of IKK-ε in a less invasive ovarian cancer cell line increased metastasis in vivo. Taken together, these data provide evidence that IKK-ε is a key coordinator of invasion and metastasis programs in ovarian cancer. Inhibition of IKK-ε signaling thus emerges as a viable therapeutic strategy in women whose ovarian cancer shows aberrant activation of this pathway. .
©2012 AACR.
Conflict of interest statement
There are no conflicts to disclose
Figures



References
-
- Pignata S, Cannella L, Leopardo D, Pisano C, Bruni GS, Facchini G. Chemotherapy in epithelial ovarian cancer. Cancer Lett. 2011;303:73–83. - PubMed
-
- Annunziata CM, Azad N, Dhamoon AS, Whiteley G, Kohn EC. Ovarian cancer in the proteomics era. Int J Gynecol Cancer. 2008;18 (Suppl 1):1–6. - PubMed
-
- Martin LP, Schilder RJ. Management of recurrent ovarian carcinoma: current status and future directions. Semin Oncol. 2009;36:112–125. - "V体育平台登录" PubMed
Publication types
- "V体育官网" Actions
- "VSports注册入口" Actions
- Actions (V体育官网)
MeSH terms
- Actions (V体育平台登录)
- Actions (V体育2025版)
- "VSports在线直播" Actions
- VSports - Actions
- VSports在线直播 - Actions
- "V体育ios版" Actions
- "VSports注册入口" Actions
- Actions (V体育平台登录)
Substances
Grants and funding
VSports注册入口 - LinkOut - more resources
"VSports最新版本" Full Text Sources
Medical
Molecular Biology Databases

