Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin
- PMID: 22927401
- PMCID: PMC3443161
- DOI: 10.1073/pnas.1203085109
Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin
Abstract
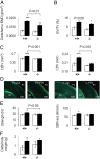
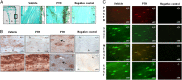
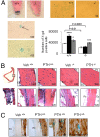
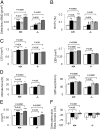
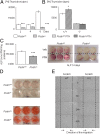
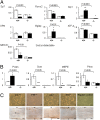
Periostin (Postn) is a matricellular protein preferentially expressed by osteocytes and periosteal osteoblasts in response to mechanical stimulation and parathyroid hormone (PTH). Whether and how periostin expression influences bone anabolism, however, remains unknown. We investigated the skeletal response of adult Postn(-/-) and Postn(+/+) mice to intermittent PTH. Compared with Postn(+/+), Postn(-/-) mice had a lower bone mass, cortical bone volume, and strength response to PTH. PTH-stimulated bone-forming indices were all significantly lower in Postn(-/-) mice, particularly at the periosteum. Furthermore, in vivo stimulation of Wnt-β-catenin signaling by PTH, as evaluated in TOPGAL reporter mice, was inhibited in the absence of periostin (TOPGAL;Postn(-/-) mice). PTH stimulated periostin and inhibited MEF2C and sclerostin (Sost) expression in bone and osteoblasts in vitro. Recombinant periostin also suppressed Sost expression, which was mediated through the integrin αVβ3 receptor, whereas periostin-blocking antibody prevented inhibition of MEF2C and Sost by PTH. In turn, administration of a Sost-blocking antiboby partially restored the PTH-mediated increase in bone mass in Postn(-/-) mice. In addition, primary osteoblasts from Postn(-/-) mice showed a lower proliferation, mineralization, and migration, both spontaneously and in response to PTH. Osteoblastic gene expression levels confirmed a defect of Postn(-/-) osteoblast differentiation with and without PTH, as well as an increased osteoblast apoptosis in the absence of periostin VSports手机版. These data elucidate the complex role of periostin on bone anabolism, through the regulation of Sost, Wnt-β-catenin signaling, and osteoblast differentiation. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kraenzlin ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7:647–656. - PubMed
-
- Kousteni S, Bilezikian JP. The cell biology of parathyroid hormone in osteoblasts. Curr Osteoporos Rep. 2008;6:72–76. - VSports - PubMed
-
- Huang JC, et al. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–244. - VSports在线直播 - PubMed
-
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. - PubMed
Publication types
- Actions (VSports注册入口)
- "VSports在线直播" Actions
MeSH terms
- VSports手机版 - Actions
- VSports在线直播 - Actions
- Actions (V体育官网入口)
- Actions (VSports)
- VSports注册入口 - Actions
- Actions (VSports手机版)
- "VSports在线直播" Actions
- VSports注册入口 - Actions
- V体育平台登录 - Actions
Substances
- Actions (VSports app下载)
- Actions (V体育2025版)
- Actions (V体育官网)
- "VSports app下载" Actions
- Actions (VSports)
- Actions (V体育ios版)
- VSports注册入口 - Actions
- VSports在线直播 - Actions
- Actions (VSports app下载)
Grants and funding
"V体育2025版" LinkOut - more resources
"V体育安卓版" Full Text Sources
Other Literature Sources (V体育安卓版)
V体育安卓版 - Medical
Molecular Biology Databases
"VSports最新版本" Miscellaneous

