"VSports手机版" Snail1 controls TGF-β responsiveness and differentiation of mesenchymal stem cells
- PMID: 22869142
- PMCID: PMC3494751 (VSports注册入口)
- DOI: "V体育平台登录" 10.1038/onc.2012.342
Snail1 controls TGF-β responsiveness and differentiation of mesenchymal stem cells
Abstract
The Snail1 transcriptional repressor plays a key role in triggering epithelial-to-mesenchymal transition. Although Snail1 is widely expressed in early development, in adult animals it is limited to a subset of mesenchymal cells where it has a largely unknown function. Using a mouse model with inducible depletion of Snail1, here we demonstrate that Snail1 is required to maintain mesenchymal stem cells (MSCs). This effect is associated to the responsiveness to transforming growth factor (TGF)-β1 that shows a strong Snail1 dependence. Snail1 depletion in conditional knockout adult animals causes a significant decrease in the number of bone marrow-derived MSCs. In culture, Snail1-deficient MSCs prematurely differentiate to osteoblasts or adipocytes and, in contrast to controls, are resistant to the TGF-β1-induced differentiation block VSports手机版. These results demonstrate a new role for Snail1 in TGF-β response and MSC maintenance. .
Figures




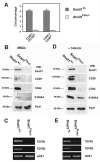


"V体育官网入口" References
-
- Peinado H, Olmeda D, Cano A. Snail, ZEB and bHLH factors in tumour progression: and alliance against the epithelial phenotype? Nat. Rev. Cancer. 2007;7:415–428. - PubMed
-
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84–89. - PubMed
-
- Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. - PubMed
-
- Solanas G, Porta-de-la-Riva M, Agustí C, Casagolda D, Sánchez-Aguilera F, Larriba MJ, et al. E-cadherin controls beta-catenin and NF-kappaB transcriptional activity in mesenchymal gene expression. J. Cell Sci. 2008;121:2224–2234. - PubMed
Publication types
- "VSports手机版" Actions
MeSH terms
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- "VSports注册入口" Actions
- V体育官网入口 - Actions
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- Actions (V体育平台登录)
- VSports在线直播 - Actions
- VSports注册入口 - Actions
- "V体育安卓版" Actions
Substances
- "V体育官网入口" Actions
- "V体育2025版" Actions
Grants and funding
V体育ios版 - LinkOut - more resources
Full Text Sources
Molecular Biology Databases
"VSports手机版" Research Materials

