New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells
- PMID: 22831834
- PMCID: PMC3570051
- DOI: 10.1093/jmcb/mjs042 (VSports手机版)
VSports注册入口 - New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells
Erratum in
-
Corrigendum to 'New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells'.J Mol Cell Biol. 2020 Nov 25;12(12):991-992. doi: 10.1093/jmcb/mjaa077. J Mol Cell Biol. 2020. PMID: 33537701 Free PMC article. No abstract available.
Abstract
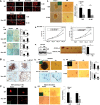
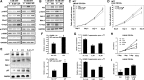
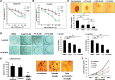
The androgen deprivation therapy (ADT) to systematically suppress/reduce androgens binding to the androgen receptor (AR) has been the standard therapy for prostate cancer (PCa); yet, most of ADT eventually fails leading to the recurrence of castration resistant PCa. Here, we found that the PCa patients who received ADT had increased PCa stem/progenitor cell population VSports手机版. The addition of the anti-androgen, Casodex, or AR-siRNA in various PCa cells led to increased stem/progenitor cells, whereas, in contrast, the addition of functional AR led to decreased stem/progenitor cell population but increased non-stem/progenitor cell population, suggesting that AR functions differentially in PCa stem/progenitor vs. non-stem/progenitor cells. Therefore, the current ADT might result in an undesired expansion of PCa stem/progenitor cell population, which explains why this therapy fails. Using various human PCa cell lines and three different mouse models, we concluded that targeting PCa non-stem/progenitor cells with AR degradation enhancer ASC-J9 and targeting PCa stem/progenitor cells with 5-azathioprine and γ-tocotrienol resulted in a significant suppression of the tumors at the castration resistant stage. This suggests that a combinational therapy that simultaneously targets both stem/progenitor and non-stem/progenitor cells will lead to better therapeutic efficacy and may become a new therapy to battle the PCa before and after castration resistant stages. .
Figures



References
-
- Bisson I., Prowse D.M. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19:683–697. - PubMed
-
- Cabrespine A., Guy L., Chollet P., et al. Molecular mechanisms involved in hormone resistance of prostate cancer. Bull. Cancer. 2004;91:747–757. - PubMed
-
- Collins A.T., Habib F.K., Maitland N.J., et al. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J. Cell Sci. 2001;114:3865–3872. - PubMed
-
- Comitato R., Nesaretnam K., Leoni G., et al. A novel mechanism of natural vitamin E tocotrienol activity: involvement of ERbeta signal transduction. Am. J. Physiol. Endocrinol. Metab. 2009;297:E427–E437. - "V体育安卓版" PubMed
-
- Crocoll A., Zhu C.C., Cato A.C., et al. Expression of androgen receptor mRNA during mouse embryogenesis. Mech. Dev. 1998;72:175–178. - PubMed (VSports app下载)
Publication types (VSports手机版)
- Actions (V体育安卓版)
MeSH terms
- V体育官网入口 - Actions
- Actions (VSports在线直播)
- VSports - Actions
- "VSports手机版" Actions
- Actions (V体育安卓版)
- "VSports最新版本" Actions
- "V体育2025版" Actions
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- Actions (VSports最新版本)
Substances
- "V体育2025版" Actions
- Actions (VSports注册入口)
- "V体育官网" Actions
- "V体育官网" Actions
VSports在线直播 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

