p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice
- PMID: 22827996
- PMCID: PMC3533440
- DOI: "V体育2025版" 10.1126/scisignal.2002918
"V体育安卓版" p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice
Abstract
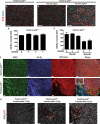
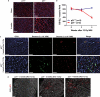
Radiation therapy, which is used for the treatment of some cancers, can cause delayed heart damage. In the heart, p53 influences myocardial injury that occurs after multiple types of stress. Here, we demonstrated that p53 functioned in endothelial cells to protect mice from myocardial injury after whole-heart irradiation. Mice with an endothelial cell-specific deletion of p53 succumbed to heart failure after whole-heart irradiation as a result of myocardial necrosis, systolic dysfunction, and cardiac hypertrophy. Moreover, the onset of cardiac dysfunction was preceded by alterations in myocardial vascular permeability and density, which resulted in cardiac ischemia and myocardial hypoxia. Mechanistic studies with primary cardiac endothelial cells irradiated in vitro indicated that p53 signaling caused mitotic arrest and protected cardiac endothelial cells from cell death resulting from abnormal mitosis or mitotic catastrophe VSports手机版. Furthermore, mice lacking the cyclin-dependent kinase inhibitor p21, which is a transcriptional target of p53, were also sensitized to myocardial injury after whole-heart irradiation. Together, our results demonstrate that the p53-p21 axis functions to prevent radiation-induced myocardial injury in mice. .
Figures (VSports在线直播)





"VSports" References
-
- Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117. - PubMed
-
- Sano M, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444. - PubMed
-
- Matsusaka H, et al. Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovas Res. 2006;70:457. - PubMed
VSports - Publication types
- VSports在线直播 - Actions
"VSports手机版" MeSH terms
- Actions (VSports在线直播)
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- Actions (V体育官网)
- "VSports" Actions
- Actions (VSports)
- Actions (V体育平台登录)
- "VSports注册入口" Actions
- VSports最新版本 - Actions
"V体育ios版" Substances
- V体育平台登录 - Actions
Grants and funding
V体育ios版 - LinkOut - more resources
Full Text Sources
Medical
V体育2025版 - Molecular Biology Databases
"VSports注册入口" Research Materials
Miscellaneous

