VSports注册入口 - New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment
- PMID: 22822400
- PMCID: PMC3398411 (V体育ios版)
- DOI: 10.3389/fphar.2012.00140
New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment
"V体育官网入口" Abstract
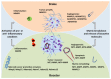
Processes such as cell proliferation, angiogenesis, apoptosis, or invasion are strongly influenced by the surrounding microenvironment of the tumor. Therefore, the ability to change these surroundings represents an important property through which tumor cells are able to acquire specific functions necessary for tumor growth and dissemination. Matrix metalloproteinases (MMPs) constitute key players in this process, allowing tumor cells to modify the extracellular matrix (ECM) and release cytokines, growth factors, and other cell-surface molecules, ultimately facilitating protease-dependent tumor progression. Remodeling of the ECM by collagenolytic enzymes such as MMP1, MMP8, MMP13, or the membrane-bound MT1-MMP as well as by other membrane-anchored proteases is required for invasion and recruitment of novel blood vessels VSports手机版. However, the multiple roles of the MMPs do not all fit into a simple pattern. Despite the pro-tumorigenic function of certain metalloproteinases, recent studies have shown that other members of these families, such as MMP8 or MMP11, have a protective role against tumor growth and metastasis in animal models. These studies have been further expanded by large-scale genomic analysis, revealing that the genes encoding metalloproteinases, such as MMP8, MMP27, ADAM7, and ADAM29, are recurrently mutated in specific tumors, while several ADAMTSs are epigenetically silenced in different cancers. The importance of these proteases in modifying the tumor microenvironment highlights the need for a deeper understanding of how stroma cells and the ECM can modulate tumor progression. .
Keywords: ADAM; cancer; matrix metalloproteinases; microenvironment; tumor V体育安卓版. .
"V体育2025版" Figures

References
-
- Bundesmann M. M., Wagner T. E., Chow Y. H., Altemeier W. A., Steinbach T., Schnapp L. M. (2012). Role of urokinase plasminogen activator receptor-associated protein in mouse lung. Am. J. Respir. Cell Mol. Biol. 46, 233–23910.1165/rcmb.2010-0485OC - DOI (VSports app下载) - PMC - PubMed
-
- Cal S., Obaya A. J., Llamazares M., Garabaya C., Quesada V., Lopez-Otin C. (2002). Cloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domains. Gene 283, 49–6210.1016/S0378-1119(01)00861-7 - DOI - PubMed
LinkOut - more resources
VSports注册入口 - Full Text Sources

