Cyclin K-containing kinase complexes maintain self-renewal in murine embryonic stem cells (VSports手机版)
- PMID: 22547058
- PMCID: PMC3408147
- DOI: "VSports手机版" 10.1074/jbc.M111.321760
"V体育安卓版" Cyclin K-containing kinase complexes maintain self-renewal in murine embryonic stem cells
Abstract
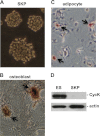
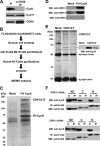
Protein phosphorylation plays an important role in the regulation of self-renewal and differentiation of embryonic stem cells. However, the responsible intracellular kinases are not well characterized VSports手机版. Here, we discovered that cyclin K protein was highly expressed in pluripotent embryonic stem cells but low in their differentiated derivatives or tissue-specific stem cells. Upon cell differentiation, the level of cyclin K protein was decreased. Furthermore, knockdown of cyclin K led to cell differentiation, which could be rescued by an expression construct resistant to RNA interference. Surprisingly, cyclin K did not interact with CDK9 protein in cells as thought previously. Instead, it associated with CrkRS (also known as CDK12) and CDC2L5 (also known as CDK13). Similar to cyclin K, both CDK12 and CDK13 proteins were highly expressed in murine embryonic stem cells and were decreased upon cell differentiation. Importantly, knockdown of either kinase resulted in differentiation. Thus, our studies have uncovered two novel protein kinase complexes that maintain self-renewal in embryonic stem cells. .
Figures




References
-
- Evans M. (2011) Discovering pluripotency: 30 years of mouse embryonic stem cells. Nat. Rev. 12, 680–686 - PubMed
-
- Young R. A. (2011) Control of the embryonic stem cell state. Cell 144, 940–954 - PMC (VSports app下载) - PubMed
-
- Saxe J. P., Tomilin A., Schöler H. R., Plath K., Huang J. (2009) Post-translational regulation of Oct4 transcriptional activity. PloS One 4, e4467. - PMC (VSports手机版) - PubMed
Publication types
- "V体育官网" Actions
MeSH terms
- Actions (VSports)
- "VSports手机版" Actions
- "VSports在线直播" Actions
- "V体育官网" Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- "V体育平台登录" Actions
- "V体育ios版" Actions
- Actions (V体育平台登录)
Substances
- Actions (V体育安卓版)
- V体育平台登录 - Actions
- Actions (VSports手机版)
LinkOut - more resources
"V体育2025版" Full Text Sources
Other Literature Sources
Molecular Biology Databases
VSports手机版 - Miscellaneous

