Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application
- PMID: 22544865
- PMCID: "V体育2025版" PMC3400832
- DOI: 10.1124/pr.111.005538
Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application
"V体育官网入口" Abstract
Aldehyde dehydrogenases (ALDHs) belong to a superfamily of enzymes that play a key role in the metabolism of aldehydes of both endogenous and exogenous derivation. The human ALDH superfamily comprises 19 isozymes that possess important physiological and toxicological functions. The ALDH1A subfamily plays a pivotal role in embryogenesis and development by mediating retinoic acid signaling. ALDH2, as a key enzyme that oxidizes acetaldehyde, is crucial for alcohol metabolism. ALDH1A1 and ALDH3A1 are lens and corneal crystallins, which are essential elements of the cellular defense mechanism against ultraviolet radiation-induced damage in ocular tissues. Many ALDH isozymes are important in oxidizing reactive aldehydes derived from lipid peroxidation and thereby help maintain cellular homeostasis VSports手机版. Increased expression and activity of ALDH isozymes have been reported in various human cancers and are associated with cancer relapse. As a direct consequence of their significant physiological and toxicological roles, inhibitors of the ALDH enzymes have been developed to treat human diseases. This review summarizes known ALDH inhibitors, their mechanisms of action, isozyme selectivity, potency, and clinical uses. The purpose of this review is to 1) establish the current status of pharmacological inhibition of the ALDHs, 2) provide a rationale for the continued development of ALDH isozyme-selective inhibitors, and 3) identify the challenges and potential therapeutic rewards associated with the creation of such agents. .
Figures




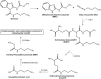
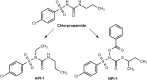
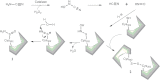
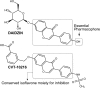
References
-
- Alison MR, Guppy NJ, Lim SM, Nicholson LJ. (2010) Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose? J Pathol 222:335–344 - "VSports最新版本" PubMed
-
- Alnouti Y, Klaassen CD. (2008) Tissue distribution, ontogeny, and regulation of aldehyde dehydrogenase (Aldh) enzymes mRNA by prototypical microsomal enzyme inducers in mice. Toxicol Sci 101:51–64 - VSports - PubMed
-
- Alter BP, Joenje H, Oostra AB, Pals G. (2005) Fanconi anemia: adult head and neck cancer and hematopoietic mosaicism. Arch Otolaryngol Head Neck Surg 131:635–639 - PubMed
"V体育官网" Publication types
- "VSports app下载" Actions
MeSH terms
- VSports - Actions
- "VSports" Actions
- VSports - Actions
- "VSports手机版" Actions
- "V体育官网入口" Actions
- "V体育官网入口" Actions
Substances
- VSports在线直播 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources (VSports)
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

