"VSports手机版" The critical role of Notch ligand Delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection
- PMID: 22072963
- PMCID: "V体育官网" PMC3207886
- DOI: 10.1371/journal.ppat.1002341
The critical role of Notch ligand Delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection
"VSports在线直播" Abstract
Influenza A viral infections have been identified as the etiologic agents for historic pandemics, and contribute to the annual mortality associated with acute viral pneumonia. While both innate and acquired immunity are important in combating influenza virus infection, the mechanism connecting these arms of the immune system remains unknown. Recent data have indicated that the Notch system is an important bridge between antigen-presenting cells (APCs) and T cell communication circuits and plays a central role in driving the immune system to overcome disease. In the present study, we examine the role of Notch signaling during influenza H1N1 virus infection, focusing on APCs. We demonstrate here that macrophages, but not dendritic cells (DCs), increased Notch ligand Delta-like 1 (Dll1) expression following influenza virus challenge VSports手机版. Dll1 expression on macrophages was dependent on retinoic acid-inducible gene-I (RIG-I) induced type-I IFN pathway, and not on the TLR3-TRIF pathway. We also found that IFNα-Receptor knockout mice failed to induce Dll1 expression on lung macrophages and had enhanced mortality during influenza virus infection. Our results further showed that specific neutralization of Dll1 during influenza virus challenge induced higher mortality, impaired viral clearance, and decreased levels of IFN-γ. In addition, we blocked Notch signaling by using γ-secretase inhibitor (GSI), a Notch signaling inhibitor. Intranasal administration of GSI during influenza infection also led to higher mortality, and higher virus load with excessive inflammation and an impaired production of IFN-γ in lungs. Moreover, Dll1 expression on macrophages specifically regulates IFN-γ levels from CD4(+)and CD8(+)T cells, which are important for anti-viral immunity. Together, the results of this study show that Dll1 positively influences the development of anti-viral immunity, and may provide mechanistic approaches for modifying and controlling the immune response against influenza H1N1 virus infection. .
Conflict of interest statement
The authors have declared that no competing interests exist.
VSports手机版 - Figures

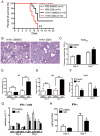
 ) (B) were stimulated with H1N1 (MOI = 10) for 6 hours, then quantitative real-time PCR was performed and the expression levels of Notch ligands were evaluated. BMDC (CD11b+CD11c+) (C) and BMDM (CD11b+F4/80+) (D) were stimulated with PolyI:C (10 µg/ml), LPS (1 μg/ml), CpG (1 µM), or H1N1 (MOI = 10) for 24 hours, then flow cytometry was performed as indicated in MATERIALS and METHODS. *P<0.05, **P<0.01 compared with PBS-treated cells. Data shown indicate mean±SEM and are from a representative experiment of 3 independent experiments. Each time point represents at least 4 mice per group. N.D. = No Detection, MFI = Mean Fluorescence Intensity.
) (B) were stimulated with H1N1 (MOI = 10) for 6 hours, then quantitative real-time PCR was performed and the expression levels of Notch ligands were evaluated. BMDC (CD11b+CD11c+) (C) and BMDM (CD11b+F4/80+) (D) were stimulated with PolyI:C (10 µg/ml), LPS (1 μg/ml), CpG (1 µM), or H1N1 (MOI = 10) for 24 hours, then flow cytometry was performed as indicated in MATERIALS and METHODS. *P<0.05, **P<0.01 compared with PBS-treated cells. Data shown indicate mean±SEM and are from a representative experiment of 3 independent experiments. Each time point represents at least 4 mice per group. N.D. = No Detection, MFI = Mean Fluorescence Intensity.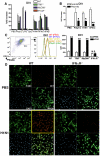



References
-
- Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–87. - PubMed
-
- Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. - PubMed
-
- Swedish KA, Conenello G, Factor SH. First Season of 2009 H1N1 Influenza. Mt Sinai J Med. 2010;77:103–113. - PubMed
-
- Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. - PubMed
"V体育2025版" Publication types
- "VSports注册入口" Actions
MeSH terms
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- "VSports app下载" Actions
- Actions (V体育ios版)
- V体育官网入口 - Actions
- VSports app下载 - Actions
- VSports在线直播 - Actions
- Actions (VSports在线直播)
- "VSports在线直播" Actions
- "V体育官网入口" Actions
- "VSports手机版" Actions
- VSports - Actions
- Actions (V体育官网入口)
- "V体育平台登录" Actions
- VSports - Actions
- V体育安卓版 - Actions
Substances
- VSports在线直播 - Actions
- V体育官网 - Actions
- "V体育ios版" Actions
- "VSports注册入口" Actions
- VSports手机版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"VSports" Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

