"VSports手机版" Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer
- PMID: 22010023
- PMCID: PMC3221526
- DOI: "VSports app下载" 10.1200/JCO.2010.34.4879
Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer
Abstract
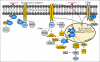
Although antiestrogen therapies targeting estrogen receptor (ER) α signaling prevent disease recurrence in the majority of patients with hormone-dependent breast cancer, a significant fraction of patients exhibit de novo or acquired resistance. Currently, the only accepted mechanism linked with endocrine resistance is amplification or overexpression of the ERBB2 (human epidermal growth factor receptor 2 [HER2]) proto-oncogene. Experimental and clinical evidence suggests that hyperactivation of the phosphatidylinositol 3-kinase (PI3K) pathway, the most frequently mutated pathway in breast cancer, promotes antiestrogen resistance. PI3K is a major signaling hub downstream of HER2 and other receptor tyrosine kinases. PI3K activates several molecules involved in cell-cycle progression and survival, and in ER-positive breast cancer cells, it promotes estrogen-dependent and -independent ER transcriptional activity. Preclinical tumor models of antiestrogen-resistant breast cancer often remain sensitive to estrogens and PI3K inhibition, suggesting that simultaneous targeting of the PI3K and ER pathways may be most effective. Herein, we review alterations in the PI3K pathway associated with resistance to endocrine therapy, the state of clinical development of PI3K inhibitors, and strategies for the clinical investigation of such drugs in hormone receptor-positive breast cancer. VSports手机版.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. - "VSports" PubMed
-
- Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. - PubMed
-
- Ellis MJ, Tao Y, Young O, et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol. 2006;24:3019–3025. - PubMed
-
- Arpino G, Green SJ, Allred DC, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: A southwest oncology group study. Clin Cancer Res. 2004;10:5670–5676. - PubMed
-
- De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. - PubMed
Publication types
- Actions (VSports)
- Actions (V体育2025版)
- Actions (V体育平台登录)
MeSH terms
- "VSports在线直播" Actions
- V体育官网入口 - Actions
- VSports在线直播 - Actions
- Actions (VSports注册入口)
- Actions (VSports app下载)
"V体育平台登录" Substances
- VSports app下载 - Actions
- Actions (VSports)
- "VSports手机版" Actions
Grants and funding
V体育ios版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
VSports手机版 - Medical
Research Materials
VSports app下载 - Miscellaneous

