A dual role of BRCA1 in two distinct homologous recombination mediated repair in response to replication arrest
- PMID: 21954437
- PMCID: PMC3258139
- DOI: 10.1093/nar/gkr748
A dual role of BRCA1 in two distinct homologous recombination mediated repair in response to replication arrest
Abstract
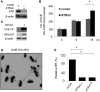
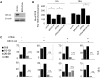
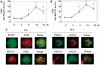
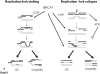
Homologous recombination (HR) is a major mechanism utilized to repair blockage of DNA replication forks. Here, we report that a sister chromatid exchange (SCE) generated by crossover-associated HR efficiently occurs in response to replication fork stalling before any measurable DNA double-strand breaks (DSBs). Interestingly, SCE produced by replication fork collapse following DNA DSBs creation is specifically suppressed by ATR, a central regulator of the replication checkpoint. BRCA1 depletion leads to decreased RPA2 phosphorylation (RPA2-P) following replication fork stalling but has no obvious effect on RPA2-P following replication fork collapse. Importantly, we found that BRCA1 promotes RAD51 recruitment and SCE induced by replication fork stalling independent of ATR. In contrast, BRCA1 depletion leads to a more profound defect in RAD51 recruitment and SCE induced by replication fork collapse when ATR is depleted. We concluded that BRCA1 plays a dual role in two distinct HR-mediated repair upon replication fork stalling and collapse. Our data established a molecular basis for the observation that defective BRCA1 leads to a high sensitivity to agents that cause replication blocks without being associated with DSBs, and also implicate a novel mechanism by which loss of cell cycle checkpoints promotes BRCA1-associated tumorigenesis via enhancing HR defect resulting from BRCA1 deficiency. VSports手机版.
Figures
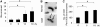
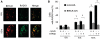


References
-
- Helleday T. Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis. 2010;31:955–960. - "V体育平台登录" PubMed
-
- Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA repair. 2007;6:923–935. - PubMed
-
- Wyman C, Kanaar R. DNA double-strand break repair: all's well that ends well. Ann. Rev. Genet. 2006;40:363–383. - PubMed
-
- Helleday T. Pathways for mitotic homologous recombination in mammalian cells. Mutat. Res. 2003;532:103–115. - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms
- Actions (V体育ios版)
- "V体育ios版" Actions
- Actions (VSports最新版本)
- Actions (VSports app下载)
- "V体育平台登录" Actions
Substances
- V体育官网入口 - Actions
- VSports手机版 - Actions
- VSports app下载 - Actions
- Actions (V体育官网入口)
Grants and funding
LinkOut - more resources
"VSports在线直播" Full Text Sources
Research Materials
Miscellaneous

