Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells
- PMID: 21941373
- PMCID: PMC3246527
- DOI: 10.1038/cdd.2011.123
"V体育2025版" Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells
"VSports手机版" Abstract
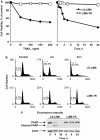
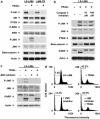
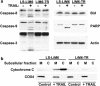
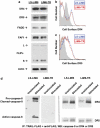
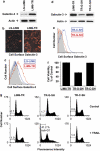
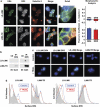
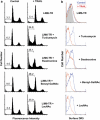
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis and preferentially kills tumor cells by engaging specific glycosylated death receptors, resulting in the internalization of ligand/receptor complexes and recruitment of the initiator caspase-8 to an activation platform known as the death-inducing signaling complex (DISC). However, emergence of TRAIL-resistant sub-populations may contribute to therapeutic failure. To investigate resistance mechanisms, we isolated a stable TRAIL-resistant sub-population of the metastatic colon cancer cell line LS-LIM6, designated LIM6-TR. LIM6-TR cells are impaired in endocytosis of TRAIL/death receptors complexes and failed to recruit/activate caspase-8 to the DISC upon TRAIL stimulation. Differential activation of Wnt and JNK pathways is not responsible for acquisition of TRAIL resistance VSports手机版. LIM6-TR cells display a marked increase in cell-surface expression of galectin-3, an endogenous lectin, which co-localizes with and binds death receptors. Silencing of galectin-3 restores TRAIL sensitivity and promotes TRAIL-mediated endocytosis of TRAIL/death receptors complexes. Inhibitors of galectin-3 and glycosylation also re-sensitize LIM6-TR to TRAIL and restore internalization of ligand/receptors complexes. These studies identify a novel TRAIL-resistance mechanism in which galectin-3 impedes trafficking of death receptor by anchoring them in glycan nano-clusters, blocking the execution of the apoptosis signal. .
Figures (VSports最新版本)
References (V体育平台登录)
-
- Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16:1701–1708. - "VSports在线直播" PubMed
-
- Abdulghani J, El-Deiry WS. TRAIL receptor signaling and therapeutics. Expert Opin Ther Targets. 2010;14:1091–1108. - PubMed
-
- Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. - PubMed (V体育ios版)
-
- Zhang Y, Yoshida T, Zhang B. TRAIL induces endocytosis of its death receptors in MDA-MB-231 breast cancer cells. Cancer Biol Ther. 2009;8:917–922. - PubMed
Publication types (VSports)
VSports最新版本 - MeSH terms
- VSports在线直播 - Actions
- VSports手机版 - Actions
- V体育2025版 - Actions
- "V体育ios版" Actions
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- Actions (V体育2025版)
- Actions (VSports最新版本)
- "VSports注册入口" Actions
- VSports - Actions
- VSports最新版本 - Actions
- V体育官网 - Actions
- Actions (VSports注册入口)
- VSports手机版 - Actions
- Actions (VSports)
- V体育2025版 - Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- Actions (V体育ios版)
- VSports - Actions
Substances
- "VSports注册入口" Actions
Grants and funding
LinkOut - more resources
Full Text Sources (V体育2025版)
Research Materials

