Fluid forces control endothelial sprouting
- PMID: 21876168
- PMCID: PMC3174629
- DOI: 10.1073/pnas.1105316108
VSports注册入口 - Fluid forces control endothelial sprouting
Abstract
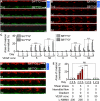
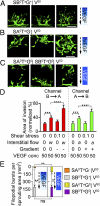
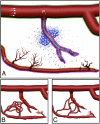
During angiogenesis, endothelial cells (ECs) from intact blood vessels quickly infiltrate avascular regions via vascular sprouting. This process is fundamental to many normal and pathological processes such as wound healing and tumor growth, but its initiation and control are poorly understood. Vascular endothelial cell growth factor (VEGF) can promote vessel dilation and angiogenic sprouting, but given the complex nature of vascular morphogenesis, additional signals are likely necessary to determine, for example, which vessel segments sprout, which dilate, and which remain quiescent. Fluid forces exerted by blood and plasma are prime candidates that might codirect these processes, but it is not known whether VEGF cooperates with mechanical fluid forces to mediate angiogenesis. Using a microfluidic tissue analog of angiogenic sprouting, we found that fluid shear stress, such as exerted by flowing blood, attenuates EC sprouting in a nitric oxide-dependent manner and that interstitial flow, such as produced by extravasating plasma, directs endothelial morphogenesis and sprout formation VSports手机版. Furthermore, positive VEGF gradients initiated sprouting but negative gradients inhibited sprouting, promoting instead sheet-like migration analogous to vessel dilation. These results suggest that ECs integrate signals from fluid forces and local VEGF gradients to achieve such varied goals as vessel dilation and sprouting. .
Conflict of interest statement
The authors declare no conflict of interest.
VSports注册入口 - Figures


References
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
-
- Davis GE, Bayless KJ, Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec. 2002;268:252–275. - PubMed
-
- Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. - PubMed
-
- Carmeliet P, De Smet F, Loges S, Mazzone M. Branching morphogenesis and antiangiogenesis candidates: Tip cells lead the way. Nat Rev Clin Oncol. 2009;6:315–326. - PubMed
Publication types
- V体育安卓版 - Actions
MeSH terms
- VSports手机版 - Actions
- Actions (VSports)
- "VSports在线直播" Actions
- V体育官网 - Actions
- Actions (VSports注册入口)
Substances (V体育2025版)
- "VSports注册入口" Actions
- "V体育2025版" Actions
Grants and funding
VSports - LinkOut - more resources
Full Text Sources
"V体育官网入口" Other Literature Sources

