Paraoxonase-2 modulates stress response of endothelial cells to oxidized phospholipids and a bacterial quorum-sensing molecule
- PMID: 21836061
- PMCID: PMC3244174
- DOI: 10.1161/ATVBAHA.111.232827
Paraoxonase-2 modulates stress response of endothelial cells to oxidized phospholipids and a bacterial quorum-sensing molecule
VSports在线直播 - Abstract
Objective: Chronic infection has long been postulated as a stimulus for atherogenesis. Pseudomonas aeruginosa infection has been associated with increased atherosclerosis in rats, and these bacteria produce a quorum-sensing molecule 3-oxo-dodecynoyl-homoserine lactone (3OC12-HSL) that is critical for colonization and virulence. Paraoxonase 2 (PON2) hydrolyzes 3OC12-HSL and also protects against the effects of oxidized phospholipids thought to contribute to atherosclerosis. We now report the response of human aortic endothelial cells (HAECs) to 3OC12-HSL and oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (Ox-PAPC) in relation to PON2 expression. VSports手机版.
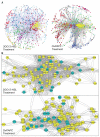
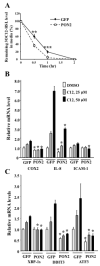
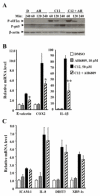
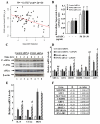
Methods and results: Using expression profiling and network modeling, we identified the unfolded protein response (UPR), cell cycle genes, and the mitogen-activated protein kinase signaling pathway to be heavily involved in the HAEC response to 3OC12-HSL V体育安卓版. The network also showed striking similarities to a network created based on HAEC response to Ox-PAPC, a major component of minimally modified low-density lipoprotein. HAECs in which PON2 was silenced by small interfering RNA showed increased proinflammatory response and UPR when treated with 3OC12-HSL or Ox-PAPC. .
Conclusion: 3OC12-HSL and Ox-PAPC influence similar inflammatory and UPR pathways. Quorum sensing molecules, such as 3OC12-HSL, contribute to the proatherogenic effects of chronic infection. The antiatherogenic effects of PON2 include destruction of quorum sensing molecules V体育ios版. .
Figures


"V体育ios版" References
-
- Stassen FR, Vainas T, Bruggeman CA. Infection and atherosclerosis. An alternative view on an outdated hypothesis. Pharmacol Rep. 2008;60:85–92. - VSports app下载 - PubMed
-
- Danesh J. Antibiotics in the prevention of heart attacks. Lancet. 2005;365:365–367. - PubMed
-
- Leinonen M, Saikku P. Evidence for infectious agents in cardiovascular disease and atherosclerosis. Lancet Infect Dis. 2002;2:11–17. - PubMed
-
- Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: An assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. - PubMed
-
- Turkay C, Saba R, Sahin N, Altunbas H, Ozbudak O, Akkaya B, Ozbilim G, Colbasi I, Turkay M, Ogunc D, Bayezid O. Effect of chronic pseudomonas aeruginosa infection on the development of atherosclerosis in a rat model. Clin Microbiol Infect. 2004;10:705–708. - "V体育ios版" PubMed
Publication types
- "VSports app下载" Actions
"V体育ios版" MeSH terms
- Actions (VSports)
- VSports手机版 - Actions
- "VSports app下载" Actions
- V体育官网入口 - Actions
- Actions (V体育平台登录)
- VSports app下载 - Actions
- Actions (VSports app下载)
- V体育2025版 - Actions
- Actions (VSports在线直播)
- Actions (V体育官网)
- "VSports在线直播" Actions
- V体育ios版 - Actions
- Actions (V体育2025版)
- V体育ios版 - Actions
Substances
- "VSports注册入口" Actions
- Actions (V体育安卓版)
- Actions (VSports注册入口)
- "VSports" Actions
- Actions (VSports注册入口)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

