Benchmarking and analysis of protein docking performance in Rosetta v3.2
- PMID: 21829626
- PMCID: PMC3149062
- DOI: 10.1371/journal.pone.0022477 (VSports最新版本)
Benchmarking and analysis of protein docking performance in Rosetta v3.2
Abstract
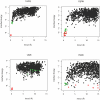
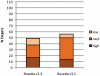
RosettaDock has been increasingly used in protein docking and design strategies in order to predict the structure of protein-protein interfaces. Here we test capabilities of RosettaDock 3. 2, part of the newly developed Rosetta v3. 2 modeling suite, against Docking Benchmark 3. 0, and compare it with RosettaDock v2 VSports手机版. 3, the latest version of the previous Rosetta software package. The benchmark contains a diverse set of 116 docking targets including 22 antibody-antigen complexes, 33 enzyme-inhibitor complexes, and 60 'other' complexes. These targets were further classified by expected docking difficulty into 84 rigid-body targets, 17 medium targets, and 14 difficult targets. We carried out local docking perturbations for each target, using the unbound structures when available, in both RosettaDock v2. 3 and v3. 2. Overall the performances of RosettaDock v2. 3 and v3. 2 were similar. RosettaDock v3. 2 achieved 56 docking funnels, compared to 49 in v2. 3. A breakdown of docking performance by protein complex type shows that RosettaDock v3. 2 achieved docking funnels for 63% of antibody-antigen targets, 62% of enzyme-inhibitor targets, and 35% of 'other' targets. In terms of docking difficulty, RosettaDock v3. 2 achieved funnels for 58% of rigid-body targets, 30% of medium targets, and 14% of difficult targets. For targets that failed, we carry out additional analyses to identify the cause of failure, which showed that binding-induced backbone conformation changes account for a majority of failures. We also present a bootstrap statistical analysis that quantifies the reliability of the stochastic docking results. Finally, we demonstrate the additional functionality available in RosettaDock v3. 2 by incorporating small-molecules and non-protein co-factors in docking of a smaller target set. This study marks the most extensive benchmarking of the RosettaDock module to date and establishes a baseline for future research in protein interface modeling and structure prediction. .
"V体育官网入口" Conflict of interest statement
"VSports最新版本" Figures

References
-
- Lensink MF, Wodak SJ. Blind predictions of protein interfaces by docking calculations in CAPRI. Proteins. 2010;78:3085–3095. - PubMed
-
- Lensink MF, Wodak SJ. Docking and scoring protein interactions: CAPRI 2009. Proteins. 2010;78:3073–3084. - "VSports手机版" PubMed
-
- Wiehe K, Pierce B, Tong WW, Hwang H, Mintseris J, et al. The performance of ZDOCK and ZRANK in rounds 6-11 of CAPRI. Proteins. 2007;69:719–725. - VSports - PubMed
-
- Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics. 2004;20:45–50. - PubMed
-
- Dominguez C, Boelens R, Bonvin AM. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125:1731–1737. - PubMed
Publication types
V体育平台登录 - MeSH terms
- "V体育ios版" Actions
- VSports最新版本 - Actions
VSports app下载 - Substances
- VSports手机版 - Actions
Grants and funding
"VSports app下载" LinkOut - more resources
"V体育ios版" Full Text Sources

