Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice
- PMID: 21266779
- PMCID: PMC3026724
- DOI: 10.1172/JCI43757
Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice
Abstract (V体育官网)
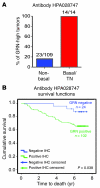
Systemic instigation is a process by which endocrine signals sent from certain tumors (instigators) stimulate BM cells (BMCs), which are mobilized into the circulation and subsequently foster the growth of otherwise indolent carcinoma cells (responders) residing at distant anatomical sites. The identity of the BMCs and their specific contribution or contributions to responder tumor growth have been elusive. Here, we have demonstrated that Sca1+ cKit- hematopoietic BMCs of mouse hosts bearing instigating tumors promote the growth of responding tumors that form with a myofibroblast-rich, desmoplastic stroma. Such stroma is almost always observed in malignant human adenocarcinomas and is an indicator of poor prognosis. We then identified granulin (GRN) as the most upregulated gene in instigating Sca1+ cKit- BMCs relative to counterpart control cells. The GRN+ BMCs that were recruited to the responding tumors induced resident tissue fibroblasts to express genes that promoted malignant tumor progression; indeed, treatment with recombinant GRN alone was sufficient to promote desmoplastic responding tumor growth. Further, analysis of tumor tissues from a cohort of breast cancer patients revealed that high GRN expression correlated with the most aggressive triple-negative, basal-like tumor subtype and reduced patient survival VSports手机版. Our data suggest that GRN and the unique hematopoietic BMCs that produce it might serve as novel therapeutic targets. .
Figures







"VSports注册入口" Comment in
-
"V体育ios版" Growing a tumor stroma: a role for granulin and the bone marrow.J Clin Invest. 2011 Feb;121(2):516-9. doi: 10.1172/JCI46088. Epub 2011 Jan 25. J Clin Invest. 2011. PMID: 21266770 Free PMC article.
References
-
- Schoenberg BS. Multiple primary malignant neoplasms. The Connecticut experience, 1935–1964. Recent Results Cancer Res. 1977;58:1–173. - PubMed
-
- Watanabe S, et al. Multiple primary cancers in 5,456 autopsy cases in the National Cancer Center of Japan. J Natl Cancer Inst. 1984;72(5):1021–1027. - PubMed
Publication types (VSports注册入口)
MeSH terms
- Actions (VSports最新版本)
- Actions (VSports手机版)
- Actions (V体育安卓版)
- Actions (VSports手机版)
- VSports手机版 - Actions
- "VSports app下载" Actions
- V体育2025版 - Actions
- VSports app下载 - Actions
Substances
Grants and funding
LinkOut - more resources (V体育ios版)
"VSports app下载" Full Text Sources
"V体育平台登录" Other Literature Sources
Medical
Molecular Biology Databases
V体育安卓版 - Miscellaneous

