"VSports app下载" Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers
- PMID: 21127264
- PMCID: PMC3009830
- DOI: 10.1073/pnas.1015245107
"VSports注册入口" Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers
Abstract
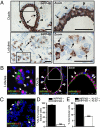
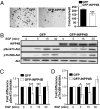
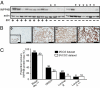
Inositol polyphosphate 4-phosphatase-II (INPP4B) is a regulator of the phosphoinositide 3-kinase (PI3K) signaling pathway and is implicated as a tumor suppressor in epithelial carcinomas. INPP4B loss of heterozygosity (LOH) is detected in some human breast cancers; however, the expression of INPP4B protein in breast cancer subtypes and the normal breast is unknown. We report here that INPP4B is expressed in nonproliferative estrogen receptor (ER)-positive cells in the normal breast, and in ER-positive, but not negative, breast cancer cell lines VSports手机版. INPP4B knockdown in ER-positive breast cancer cells increased Akt activation, cell proliferation, and xenograft tumor growth. Conversely, reconstitution of INPP4B expression in ER-negative, INPP4B-null human breast cancer cells reduced Akt activation and anchorage-independent growth. INPP4B protein expression was frequently lost in primary human breast carcinomas, associated with high clinical grade and tumor size and loss of hormone receptors and was lost most commonly in aggressive basal-like breast carcinomas. INPP4B protein loss was also frequently observed in phosphatase and tensin homolog (PTEN)-null tumors. These studies provide evidence that INPP4B functions as a tumor suppressor by negatively regulating normal and malignant mammary epithelial cell proliferation through regulation of the PI3K/Akt signaling pathway, and that loss of INPP4B protein is a marker of aggressive basal-like breast carcinomas. .
Conflict of interest statement
The authors declare no conflict of interest.
"V体育平台登录" Figures


References
-
- Ma K, Cheung SM, Marshall AJ, Duronio V. PI(3,4,5)P3 and PI(3,4)P2 levels correlate with PKB/akt phosphorylation at Thr308 and Ser473, respectively; PI(3,4)P2 levels determine PKB activity. Cell Signal. 2008;20:684–694. - PubMed
-
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. - "V体育官网入口" PubMed
-
- Scheid MP, et al. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: Studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J Biol Chem. 2002;277:9027–9035. - VSports - PubMed
-
- Norris FA, Majerus PW. Hydrolysis of phosphatidylinositol 3,4-bisphosphate by inositol polyphosphate 4-phosphatase isolated by affinity elution chromatography. J Biol Chem. 1994;269:8716–8720. - PubMed
Publication types
- Actions (V体育安卓版)
MeSH terms
- VSports最新版本 - Actions
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- Actions (V体育官网入口)
- VSports在线直播 - Actions
- VSports - Actions
- V体育官网入口 - Actions
- "VSports app下载" Actions
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- Actions (V体育官网入口)
Substances
- "VSports" Actions
- Actions (VSports注册入口)
- "VSports app下载" Actions
- "VSports最新版本" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

