MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition
- PMID: 21102519
- PMCID: PMC3063863
- DOI: 10.1038/onc.2010.526
"VSports app下载" MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition
"VSports" Abstract
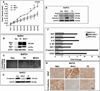
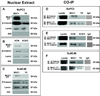
Increased motility and invasiveness of pancreatic cancer cells are associated with epithelial to mesenchymal transition (EMT). Snai1 and Slug are zinc-finger transcription factors that trigger this process by repressing E-cadherin and enhancing vimentin and N-cadherin protein expression. However, the mechanisms that regulate this activation in pancreatic tumors remain elusive. MUC1, a transmembrane mucin glycoprotein, is associated with the most invasive forms of pancreatic ductal adenocarcinomas (PDA). In this study, we show that over expression of MUC1 in pancreatic cancer cells triggers the molecular process of EMT, which translates to increased invasiveness and metastasis. EMT was significantly reduced when MUC1 was genetically deleted in a mouse model of PDA or when all seven tyrosines in the cytoplasmic tail of MUC1 were mutated to phenylalanine (mutated MUC1 CT). Using proteomics, RT-PCR and western blotting, we revealed a significant increase in vimentin, Slug and Snail expression with repression of E-Cadherin in MUC1-expressing cells compared with cells expressing the mutated MUC1 CT VSports手机版. In the cells that carried the mutated MUC1 CT, MUC1 failed to co-immunoprecipitate with β-catenin and translocate to the nucleus, thereby blocking transcription of the genes associated with EMT and metastasis. Thus, functional tyrosines are critical in stimulating the interactions between MUC1 and β-catenin and their nuclear translocation to initiate the process of EMT. This study signifies the oncogenic role of MUC1 CT and is the first to identify a direct role of the MUC1 in initiating EMT during pancreatic cancer. The data may have implications in future design of MUC1-targeted therapies for pancreatic cancer. .
Conflict of interest statement
Drs. Mukherjee and Gendler work has been funded by the NIH. There are no other conflicts to declare V体育安卓版. All other authors declare no conflicts of interests.
Figures (VSports app下载)



References
-
- Chang BW, Siccion E, Saif MW. Updates in locally advanced pancreatic cancer. Highlights from the "2010 ASCO Annual Meeting". Chicago, IL, USA. June 4–8, 2010. JOP. 2010;11:313–316. - "V体育官网入口" PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. - PubMed
-
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. - PubMed
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. - PubMed
Publication types
MeSH terms
- "VSports注册入口" Actions
- VSports在线直播 - Actions
- Actions (V体育安卓版)
- "VSports手机版" Actions
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- Actions (V体育安卓版)
- V体育官网 - Actions
- V体育平台登录 - Actions
- "V体育安卓版" Actions
- "VSports app下载" Actions
- "VSports手机版" Actions
- "VSports注册入口" Actions
Substances
- "V体育ios版" Actions
- "V体育2025版" Actions
- Actions (VSports app下载)
- VSports app下载 - Actions
- Actions (V体育2025版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育2025版)
Medical
Research Materials
Miscellaneous

