Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss
- PMID: 20543949
- PMCID: PMC2882947
- DOI: 10.1371/journal.pone.0011033
V体育安卓版 - Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss
Abstract (VSports手机版)
Background: In obesity, impaired adipose tissue function may promote secondary disease through ectopic lipid accumulation and excess release of adipokines, resulting in systemic low-grade inflammation, insulin resistance and organ dysfunction. However, several of the genes regulating adipose tissue function in obesity are yet to be identified. VSports手机版.
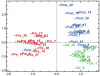
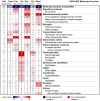
Methodology/principal findings: In order to identify novel candidate genes that may regulate adipose tissue function, we analyzed global gene expression in abdominal subcutaneous adipose tissue before and one year after bariatric surgery (biliopancreatic diversion with duodenal switch, BPD/DS) (n = 16). Adipose tissue from lean healthy individuals was also analyzed (n = 13) V体育安卓版. Two different microarray platforms (AB 1700 and Illumina) were used to measure the differential gene expression, and the results were further validated by qPCR. Surgery reduced BMI from 53. 3 to 33. 1 kg/m(2). The majority of differentially expressed genes were down-regulated after profound fat loss, including transcription factors involved in stress response, inflammation, and immune cell function (e. g. , FOS, JUN, ETS, C/EBPB, C/EBPD). Interestingly, a distinct set of genes was up-regulated after fat loss, including homeobox transcription factors (IRX3, IRX5, HOXA5, HOXA9, HOXB5, HOXC6, EMX2, PRRX1) and extracellular matrix structural proteins (COL1A1, COL1A2, COL3A1, COL5A1, COL6A3). .
Conclusions/significance: The data demonstrate a marked switch of transcription factors in adipose tissue after profound fat loss, providing new molecular insight into a dichotomy between stress response and metabolically favorable tissue development. Our findings implicate homeobox transcription factors as important regulators of adipose tissue function. V体育ios版.
Conflict of interest statement
Figures

References
-
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. - PMC (VSports最新版本) - PubMed
-
- Poulos SP, Hausman DB, Hausman GJ. The development and endocrine functions of adipose tissue. Mol Cell Endocrinol 2009 - PubMed (V体育ios版)
-
- Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343–368. - PubMed
-
- Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117:241–250. - PubMed
-
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. - PubMed
Publication types
- Actions (V体育ios版)
MeSH terms
- V体育ios版 - Actions
- "VSports" Actions
- Actions (VSports)
- "V体育ios版" Actions
Substances
- "VSports在线直播" Actions
LinkOut - more resources
V体育官网 - Full Text Sources
Other Literature Sources
Medical
"VSports在线直播" Research Materials
"VSports app下载" Miscellaneous

