The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells
- PMID: 20467795
- PMCID: PMC3721368
- DOI: 10.1007/s10911-010-9181-1 (V体育ios版)
The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells (V体育官网)
Abstract
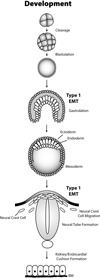
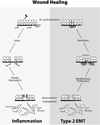
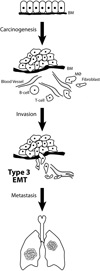
Epithelial-mesenchymal transition (EMT) is an essential process that drives polarized, immotile mammary epithelial cells (MECs) to acquire apolar, highly migratory fibroblastoid-like features. EMT is an indispensable process that is associated with normal tissue development and organogenesis, as well as with tissue remodeling and wound healing. In stark contrast, inappropriate reactivation of EMT readily contributes to the development of a variety of human pathologies, particularly those associated with tissue fibrosis and cancer cell invasion and metastasis, including that by breast cancer cells. Although metastasis is unequivocally the most lethal aspect of breast cancer and the most prominent feature associated with disease recurrence, the molecular mechanisms whereby EMT mediates the initiation and resolution of breast cancer metastasis remains poorly understood VSports手机版. Transforming growth factor-beta (TGF-beta) is a multifunctional cytokine that is intimately involved in regulating numerous physiological processes, including cellular differentiation, homeostasis, and EMT. In addition, TGF-beta also functions as a powerful tumor suppressor in MECs, whose neoplastic development ultimately converts TGF-beta into an oncogenic cytokine in aggressive late-stage mammary tumors. Recent findings have implicated the process of EMT in mediating the functional conversion of TGF-beta during breast cancer progression, suggesting that the chemotherapeutic targeting of EMT induced by TGF-beta may offer new inroads in ameliorating metastatic disease in breast cancer patients. Here we review the molecular, cellular, and microenvironmental factors that contribute to the pathophysiological activities of TGF-beta during its regulation of EMT in normal and malignant MECs. .
Figures
References
-
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. - "V体育安卓版" PMC - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. - PubMed
-
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. - PubMed
-
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. - PubMed
Publication types
- "V体育安卓版" Actions
- V体育平台登录 - Actions
MeSH terms
- Actions (V体育安卓版)
- V体育2025版 - Actions
- "V体育2025版" Actions
- Actions (VSports在线直播)
- VSports最新版本 - Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- "V体育平台登录" Actions
- "VSports最新版本" Actions
- "V体育官网入口" Actions
Substances (V体育2025版)
- Actions (VSports注册入口)
"VSports" Grants and funding
LinkOut - more resources
Full Text Sources
Medical

