Myristoylated Naked2 antagonizes Wnt-beta-catenin activity by degrading Dishevelled-1 at the plasma membrane
- PMID: 20177058
- PMCID: VSports注册入口 - PMC2859517
- DOI: "V体育安卓版" 10.1074/jbc.M109.075945
V体育官网入口 - Myristoylated Naked2 antagonizes Wnt-beta-catenin activity by degrading Dishevelled-1 at the plasma membrane
"V体育ios版" Abstract
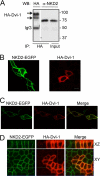
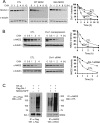
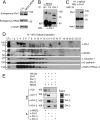
In Drosophila, naked cuticle is an inducible antagonist of the Wnt-beta-catenin pathway, likely acting at the level of Dishevelled (Dsh/Dvl), an essential component of this pathway. The mechanism by which naked cuticle and its two vertebrate orthologs, Naked1 (NKD1) and Naked2 (NKD2), inhibit Dvl function is unknown. NKD2 is myristoylated, a co-translational modification that leads to its plasma membrane localization. In contrast, myristoylation-deficient G2A NKD2 is cytoplasmic. Herein we show that the ability of Nkd2/NKD2 to antagonize Wnt-beta-catenin activity during zebrafish embryonic development and in mammalian HEK293 cells is myristoylation-dependent. NKD2 and Dvl-1 interact and co-localize at the lateral membrane of polarized epithelial cells. In reciprocal overexpression and siRNA knockdown experiments, NKD2 and Dvl-1 destabilize each other via enhanced polyubiquitylation; this effect is also dependent upon Naked2 myristoylation. Cell fractionation and ubiquitylation assays indicate that endogenous NKD2 interacts with a slower migrating, ubiquitylated form of Dvl-1 in plasma membrane fractions VSports手机版. These results provide a mechanism by which NKD2 antagonizes Wnt signaling: myristoylated NKD2 interacts with Dvl-1 at the plasma membrane, and this interaction leads to their mutual ubiquitin-mediated proteasomal degradation. .
Figures


References (VSports最新版本)
-
- Cadigan K. M., Nusse R. (1997) Genes Dev. 11, 3286–3305 - PubMed
-
- Wallingford J. B., Habas R. (2005) Development 132, 4421–4436 - "VSports手机版" PubMed
-
- Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. (1998) Nature 391, 357–362 - PubMed
-
- Takemaru K., Yamaguchi S., Lee Y. S., Zhang Y., Carthew R. W., Moon R. T. (2003) Nature 422, 905–909 - V体育安卓版 - PubMed
Publication types
MeSH terms
- Actions (V体育2025版)
- "V体育2025版" Actions
- V体育2025版 - Actions
- "V体育官网" Actions
- V体育2025版 - Actions
- VSports app下载 - Actions
- "VSports手机版" Actions
- "VSports app下载" Actions
- "VSports手机版" Actions
- "V体育官网入口" Actions
- V体育官网入口 - Actions
- Actions (VSports手机版)
Substances
- Actions (V体育官网)
- "V体育ios版" Actions
- Actions (V体育ios版)
- Actions (V体育平台登录)
- Actions (VSports手机版)
- "V体育ios版" Actions
- VSports最新版本 - Actions
- "V体育官网入口" Actions
Grants and funding
"V体育平台登录" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

