The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions
- PMID: 20054236
- PMCID: PMC2975271
- DOI: 10.4161/cc.8.24.10800
The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions
Abstract
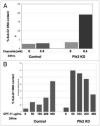
Tuberous sclerosis complex 1 (TSC1) inhibits mammalian target of rapamycin (mTOR), a central promotor of cell growth and proliferation. The protein product of the TSC1 gene, hamartin (referred to as TSC1) is known to interact with Polo-like kinase 1 (Plk1) in a cell cycle regulated, phosphorylation-dependent manner. We hypothesized that the p53 target gene, Plk2, is a tumor suppressor, mediating its tumor suppressor function through interactions with TSC1 that facilitate TSC1/2 restraint of mTOR under hypoxic stress. We found that human lung tumor cells deficient in Plk2 grew larger than control tumors, and that Plk2 interacts with endogenous TSC1 protein. Additionally, C-terminal Plk2-GST fusion protein bound both TSC1 and TSC2 proteins. TSC1 levels were elevated in response to Adriamycin and cells transiently overexpressing Plk2 demonstrated decreased phosphorylation of the downstream target of mTOR, ribosomal protein p70S6 kinase during hypoxia. Plk2 levels were inversely correlated with cytoplasmic p70S6K phosphorylation. Plk2 levels did not increase in response to DNA damage (Adriamycin, CPT -11) when HCT 116 and H460 cells were exposed to hypoxia. TSC1-deficient mouse embryonic fibroblasts with TSC1 added back demonstrated decreased S6K phosphorylation, which was further decreased when Plk2 was transiently overexpressed. Interestingly, under normoxia, Plk2 deficient tumor cells demonstrated increased apoptosis in response to various chemotherapeutic agents including CPT -11 but increased resistance to apoptotic death after CPT-11 treatment under hypoxia, and tumor xenografts comprised of these Plk2-deficient cells were resistant to CPT -11. Our results point to a novel Plk2-TSC1 interaction with effects on mTOR signaling during hypoxia, and tumor growth that may enable targeting Plk2 signaling in cancer therapy. VSports手机版.
"VSports在线直播" Figures








References (V体育官网)
-
- Guertin D, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. - PubMed
-
- Crino PB, Hathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. - PubMed
-
- Astrinidis A, Senapedis W, Henske EP. Hamartin, the tuberous sclerosis complex 1 gene product, interacts with polo-like kinase 1 in a phosphorylation-dependent manner. Human Mol Genet. 2006;15:287–97. - PubMed
-
- Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Reutz S, O’Reilly T, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damage induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. - PubMed
Publication types
- VSports最新版本 - Actions
- VSports最新版本 - Actions
MeSH terms
- V体育官网入口 - Actions
- Actions (V体育平台登录)
- V体育安卓版 - Actions
- "VSports app下载" Actions
- V体育官网 - Actions
- VSports在线直播 - Actions
- "VSports在线直播" Actions
- VSports - Actions
- "VSports" Actions
V体育ios版 - Substances
- V体育2025版 - Actions
- "V体育平台登录" Actions
- VSports在线直播 - Actions
- "VSports app下载" Actions
- "VSports手机版" Actions
- V体育平台登录 - Actions
- Actions (V体育官网)
- V体育安卓版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials (VSports手机版)
Miscellaneous (VSports手机版)
