VSports最新版本 - Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation
- PMID: 19962665
- PMCID: PMC2804903
- DOI: 10.1016/j.ccr.2009.10.016
"VSports app下载" Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation
Abstract
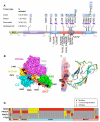
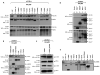
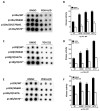
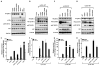
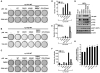
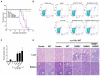
Members of the mammalian phosphoinositide-3-OH kinase (PI3K) family of proteins are critical regulators of various cellular process including cell survival, growth, proliferation, and motility. Oncogenic activating mutations in the p110alpha catalytic subunit of the heterodimeric p110/p85 PI3K enzyme are frequent in human cancers. Here we show the presence of frequent mutations in p85alpha in colon cancer, a majority of which occurs in the inter-Src homology-2 (iSH2) domain. These mutations uncouple and retain p85alpha's p110-stabilizing activity, while abrogating its p110-inhibitory activity. The p85alpha mutants promote cell survival, AKT activation, anchorage-independent cell growth, and oncogenesis in a p110-dependent manner. VSports手机版.
Figures
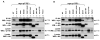
Comment in
-
V体育ios版 - PI3K regulatory subunits lose control in cancer.Cancer Cell. 2009 Dec 8;16(6):449-50. doi: 10.1016/j.ccr.2009.11.017. Cancer Cell. 2009. PMID: 19962660
References
-
- Backer JM, Myers MG, Jr., Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J, et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. Embo J. 1992;11:3469–3479. - "V体育2025版" PMC - PubMed
-
- Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. - PubMed
-
- Borlado LR, Redondo C, Alvarez B, Jimenez C, Criado LM, Flores J, Marcos MA, Martinez AC, Balomenos D, Carrera AC. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. Faseb J. 2000;14:895–903. - PubMed
Publication types
- Actions (V体育平台登录)
MeSH terms
- "V体育ios版" Actions
- Actions (VSports app下载)
- VSports - Actions
- "VSports在线直播" Actions
- "VSports app下载" Actions
Substances (V体育平台登录)
Associated data
- Actions
Grants and funding
V体育官网 - LinkOut - more resources
Full Text Sources (V体育安卓版)
V体育官网入口 - Other Literature Sources
Medical
Molecular Biology Databases (V体育ios版)
Miscellaneous

