Gonadotropin-regulated lymphangiogenesis in ovarian cancer is mediated by LEDGF-induced expression of VEGF-C
- PMID: 19934313
- PMCID: PMC2794933
- DOI: 10.1158/0008-5472.CAN-09-1213
Gonadotropin-regulated lymphangiogenesis in ovarian cancer is mediated by LEDGF-induced expression of VEGF-C (VSports)
Abstract
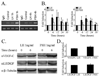
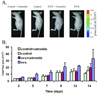
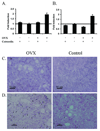
The risk and severity of ovarian carcinoma, the leading cause of gynecologic malignancy death, are significantly elevated in postmenopausal women. Ovarian failure at menopause, associated with a reduction in estrogen secretion, results in an increase of the gonadotropic luteinizing hormone (LH) and follicle-stimulating hormone (FSH), suggesting a role for these hormones in facilitating the progression of ovarian carcinoma. The current study examined the influence of hormonal stimulation on lymphangiogenesis in ovarian cancer cells. In vitro stimulation of ES2 ovarian carcinoma cells with LH and FSH induced expression of vascular endothelial growth factor (VEGF)-C. In vivo, ovariectomy of mice resulted in activation of the VEGF-C promoter in ovarian carcinoma xenografts, increased VEGF-C mRNA level, and enhanced tumor lymphangiogenesis and angiogenesis. Seeking the molecular mechanism, we examined the role of lens epithelium-derived growth factor (LEDGF/p75) and the possible contribution of its putative target, a conserved stress-response element identified in silico in the VEGF-C promoter. Using chromatin immunoprecipitation, we showed that LEDGF/p75 indeed binds the VEGF-C promoter, and binding is augmented by FSH. A corresponding hormonally regulated increase in the LEDGF/p75 mRNA and protein levels was observed. Suppression of LEDGF/p75 expression using small interfering RNA, suppression of LH and FSH production using the gonadotropin-releasing hormone antagonist cetrorelix, or mutation of the conserved stress-response element suppressed the hormonally induced expression of VEGF-C. Overall, our data suggest a possible role for elevated gonadotropins in augmenting ovarian tumor lymphangiogenesis in postmenopausal women. VSports手机版.
Figures
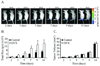
References
-
- Mandai M, Konishi I, Kuroda H, Fujii S. LH/hCG action and development of ovarian cancer--a short review on biological and clinical/epidemiological aspects. Mol Cell Endocrinol. 2007;269:61–64. - PubMed
-
- Parrott JA, Doraiswamy V, Kim G, Mosher R, Skinner MK. Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol Cell Endocrinol. 2001;172:213–222. - VSports最新版本 - PubMed
-
- Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61:6768–6776. - VSports在线直播 - PubMed
-
- Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomarkers Prev. 2005;14:98–107. - V体育官网入口 - PubMed
-
- Choi JH, Choi KC, Auersperg N, Leung PC. Gonadotropins activate proteolysis and increase invasion through protein kinase A and phosphatidylinositol 3-kinase pathways in human epithelial ovarian cancer cells. Cancer Res. 2006;66:3912–3920. - PubMed
Publication types
- "VSports" Actions
MeSH terms (V体育安卓版)
- VSports在线直播 - Actions
- V体育ios版 - Actions
- Actions (VSports注册入口)
- "V体育ios版" Actions
- Actions (V体育ios版)
- "VSports在线直播" Actions
- Actions (VSports注册入口)
- Actions (VSports在线直播)
- Actions (V体育2025版)
- Actions (VSports app下载)
- "VSports手机版" Actions
- "VSports在线直播" Actions
- "VSports在线直播" Actions
Substances
- "V体育平台登录" Actions
- "V体育官网入口" Actions
- VSports在线直播 - Actions
- VSports手机版 - Actions
Grants and funding (VSports)
LinkOut - more resources
Full Text Sources
Medical
VSports app下载 - Research Materials

