Hydrophobic and basic domains target proteins to lipid droplets
- PMID: 19874557
- PMCID: V体育官网入口 - PMC2913680
- DOI: 10.1111/j.1600-0854.2009.00994.x
"VSports" Hydrophobic and basic domains target proteins to lipid droplets
Abstract (V体育官网)
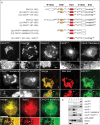
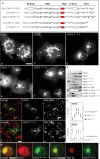
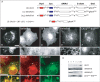
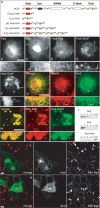
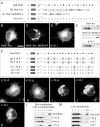
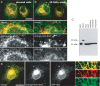
In recent years, progress in the study of the lateral organization of the plasma membrane has led to the proposal that mammalian cells use two different organelles to store lipids: intracellular lipid droplets (LDs) and plasma membrane caveolae. Experimental evidence suggests that caveolin (CAV) may act as a sensitive lipid-organizing molecule that physically connects these two lipid-storing organelles. Here, we determine the sequences necessary for efficient sorting of CAV to LDs. We show that targeting is a process cooperatively mediated by two motifs. CAV's central hydrophobic domain (Hyd) anchors CAV to the endoplasmic reticulum (ER). Next, positively charged sequences (Pos-Seqs) mediate sorting of CAVs into LDs. Our findings were confirmed by identifying an equivalent, non-conserved but functionally interchangeable Pos-Seq in ALDI, a bona fide LD-resident protein. Using this information, we were able to retarget a cytosolic protein and convert it to an LD-resident protein. Further studies suggest three requirements for targeting via this mechanism: the positive charge of the Pos-Seq, physical proximity between Pos-Seq and Hyd and a precise spatial orientation between both motifs. The study uncovers remarkable similarities with the signals that target proteins to the membrane of mitochondria and peroxisomes VSports手机版. .
"VSports注册入口" Figures
References
-
- Murphy S, Martin S, Parton RG. Lipid droplet-organelle interactions; sharing the fats. Biochim Biophys Acta. 2009;1791:441–447. - PubMed
-
- Ohsaki Y, Cheng J, Suzuki M, Shinohara Y, Fujita A, Fujimoto T. Biogenesis of cytoplasmic lipid droplets: from the lipid ester globule in the membrane to the visible structure. Biochim Biophys Acta. 2009;1791:399–407. - VSports - PubMed
-
- Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J Biol Chem. 2002;277:44507–44512. - VSports app下载 - PubMed
-
- Leber R, Zinser E, Zellnig G, Paltauf F, Daum G. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast. 1994;10:1421–1428. - PubMed
-
- Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–847. - PubMed
Publication types
- VSports在线直播 - Actions
MeSH terms
- "VSports在线直播" Actions
- V体育安卓版 - Actions
Substances
- V体育官网入口 - Actions
- Actions (VSports注册入口)
Grants and funding
"VSports" LinkOut - more resources
"VSports" Full Text Sources
"VSports最新版本" Research Materials

