S-adenosyl homocysteine hydrolase is required for Myc-induced mRNA cap methylation, protein synthesis, and cell proliferation
- PMID: 19805518
- PMCID: "V体育ios版" PMC2786700
- DOI: 10.1128/MCB.00973-09
S-adenosyl homocysteine hydrolase is required for Myc-induced mRNA cap methylation, protein synthesis, and cell proliferation
"VSports app下载" Abstract
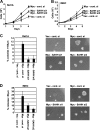
The c-Myc proto-oncogene promotes mRNA cap methylation, which is essential for almost all mRNA translation. The mRNA cap methylation reaction produces an inhibitory byproduct, S-adenosyl homocysteine. Here we report that Myc promotes upregulation of S-adenosyl homocysteine hydrolase (SAHH), an enzyme which hydrolyzes S-adenosyl homocysteine, thus neutralizing its inhibitory effects, and this is required for c-Myc-induced mRNA cap methylation. c-Myc-induced mRNA cap methylation was repressed by inhibiting the expression or activity of SAHH, whereas the same treatments did not have a significant effect on c-Myc-induced transcription or other c-Myc-dependent methylation events. The selective inhibition of mRNA cap methylation afforded by SAHH repression revealed that c-Myc-induced cap methylation could be correlated with the core c-Myc functions of protein synthesis, cell proliferation, and cell transformation VSports手机版. .
"V体育官网" Figures


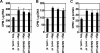


References
-
- Bentley, D. L. 2005. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr. Opin. Cell Biol. 17:251-256. - PubMed
-
- Brenner, C., R. Deplus, C. Didelot, A. Loriot, E. Vire, C. De Smet, A. Gutierrez, D. Danovi, D. Bernard, T. Boon, P. G. Pelicci, B. Amati, T. Kouzarides, Y. de Launoit, L. Di Croce, and F. Fuks. 2005. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 24:336-346. - V体育2025版 - PMC - PubMed
-
- Chiang, P. K., R. K. Gordon, J. Tal, G. C. Zeng, B. P. Doctor, K. Pardhasaradhi, and P. P. McCann. 1996. S-Adenosylmethionine and methylation. FASEB J. 10:471-480. - PubMed
-
- Cole, M. D., and V. H. Cowling. 2009. Specific regulation of mRNA cap methylation by the c-Myc and E2F1 transcription factors. Oncogene 28:1169-1175. - "VSports注册入口" PMC - PubMed
Publication types
MeSH terms
- "VSports注册入口" Actions
- "V体育ios版" Actions
- Actions (VSports注册入口)
- "V体育ios版" Actions
- Actions (VSports app下载)
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
