SBDS expression and localization at the mitotic spindle in human myeloid progenitors (VSports在线直播)
- PMID: 19759903
- PMCID: V体育安卓版 - PMC2738965
- DOI: V体育官网 - 10.1371/journal.pone.0007084
SBDS expression and localization at the mitotic spindle in human myeloid progenitors
Abstract
Background: Shwachman-Diamond Syndrome (SDS) is a hereditary disease caused by mutations in the SBDS gene. SDS is clinically characterized by pancreatic insufficiency, skeletal abnormalities and bone marrow dysfunction. The hematologic abnormalities include neutropenia, neutrophil chemotaxis defects, and an increased risk of developing Acute Myeloid Leukemia (AML). Although several studies have suggested that SBDS as a protein plays a role in ribosome processing/maturation, its impact on human neutrophil development and function remains to be clarified VSports手机版. .
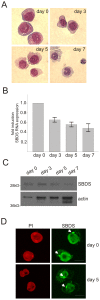
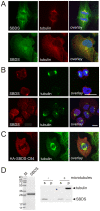
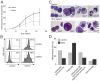
Methodology/principal findings: We observed that SBDS RNA and protein are expressed in the human myeloid leukemia PLB-985 cell line and in human hematopoietic progenitor cells by quantitative RT-PCR and Western blot analysis. SBDS expression is downregulated during neutrophil differentiation. Additionally, we observed that the differentiation and proliferation capacity of SDS-patient bone marrow hematopoietic progenitor cells in a liquid differentiation system was reduced as compared to control cultures. Immunofluorescence analysis showed that SBDS co-localizes with the mitotic spindle and in vitro binding studies reveal a direct interaction of SBDS with microtubules V体育安卓版. In interphase cells a perinuclear enrichment of SBDS protein which co-localized with the microtubule organizing center (MTOC) was observed. Also, we observed that transiently expressed SDS patient-derived SBDS-K62 or SBDS-C84 mutant proteins could co-localize with the MTOC and mitotic spindle. .
Conclusions/significance: SBDS co-localizes with the mitotic spindle, suggesting a role for SBDS in the cell division process, which corresponds to the decreased proliferation capacity of SDS-patient bone marrow CD34(+) hematopoietic progenitor cells in our culture system and also to the neutropenia in SDS patients V体育ios版. A role in chromosome missegregation has not been clarified, since similar spatial and time-dependent localization is observed when patient-derived SBDS mutant proteins are studied. Thus, the increased risk of myeloid malignancy in SDS remains unexplained. .
"VSports app下载" Conflict of interest statement
V体育ios版 - Figures


References
-
- Shwachman H, Diamond LK, Oski FA, Khaw KT. The syndrome of pancreatic insufficiency and bone marrow dysfunction. J Pediatr. 1964;65:645–63. - PubMed
-
- Bodian M, Sheldon W, Lightwood R. Congenital hypoplasia of the exocrine pancreas. Acta Paediatr. 1964;53:282–93: 282-293. - PubMed
-
- Ginzberg H, Shin J, Ellis L, Morrison J, Ip W, et al. Shwachman syndrome: phenotypic manifestations of sibling sets and isolated cases in a large patient cohort are similar. J Pediatr. 1999;135:81–88. - PubMed
-
- Kuijpers TW, Alders M, Tool AT, Mellink C, Roos D, et al. Hematologic abnormalities in Shwachman Diamond syndrome: lack of genotype-phenotype relationship. Blood. 2005;106:356–361. - PubMed
-
- Shimamura A. Shwachman-Diamond Syndrome. Semin Hematol. 2006;43:178–188. - PubMed
Publication types
MeSH terms (V体育安卓版)
- "V体育官网" Actions
- Actions (VSports注册入口)
- VSports最新版本 - Actions
- V体育2025版 - Actions
- "V体育ios版" Actions
- "VSports手机版" Actions
Substances
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- "V体育安卓版" Actions
LinkOut - more resources
Full Text Sources
"VSports在线直播" Medical
Molecular Biology Databases

