Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells
- PMID: 19432400
- PMCID: PMC2747638
- DOI: 10.1021/pr9001614
Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells
Abstract
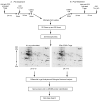
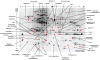
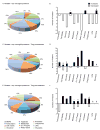
Microglial inflammatory responses affect Parkinson's disease (PD) associated nigrostriatal degeneration VSports手机版. This is triggered, in measure, by misfolded, nitrated alpha-synuclein (N-alpha-syn) contained within Lewy bodies that are released from dying or dead dopaminergic neurons into the extravascular space. N-alpha-syn-stimulated microglial immunity is regulated by CD4+ T cell subset. Indeed, CD4+CD25+ regulatory T cells (Treg) induce neuroprotective immune responses. This is seen in rodent models of stroke, amyotrophic lateral sclerosis, human immunodeficiency virus associated neurocognitive disorders, and PD. To elucidate the mechanism for Treg-mediated microglial neuroregulatory responses, we used a proteomic platform integrating difference gel electrophoresis and tandem mass spectrometry peptide sequencing. These tests served to determine consequences of Treg on the N-alpha-syn stimulated microglia. The data demonstrated that Treg substantially alter the microglial proteome in response to N-alpha-syn. This is seen through Treg abilities to suppress microglial proteins linked to cell metabolism, migration, protein transport and degradation, redox biology, cytoskeletal, and bioenergetic activities. We conclude that Treg modulate the N-alpha-syn microglial proteome and, in this way, can slow the tempo and course of PD. .
Figures

References
-
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909. - "V体育官网" PubMed
-
- Fahn S, Przedborski S. Parkinsonism. In: Rowland LP, editor. Merritt's Neurology. Lippincott Williams & Wilkins; New York: 2000. pp. 679–693.
-
- Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26:81–104. - PubMed
-
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11(8):493–8. - "VSports在线直播" PubMed
Publication types
MeSH terms
- "V体育官网入口" Actions
- "VSports在线直播" Actions
- V体育ios版 - Actions
- "VSports在线直播" Actions
- "VSports手机版" Actions
- Actions (V体育官网)
- "V体育2025版" Actions
- Actions (V体育官网入口)
Substances
- "V体育安卓版" Actions
Grants and funding
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01MH64570/MH/NIMH NIH HHS/United States
- "V体育官网入口" 5P01NS31492/NS/NINDS NIH HHS/United States
- "VSports app下载" P01 NS031492/NS/NINDS NIH HHS/United States
- U54 NS043011/NS/NINDS NIH HHS/United States
- VSports手机版 - 2R01 NS034239/NS/NINDS NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- U54NS43011/NS/NINDS NIH HHS/United States
- R01 NS036126/NS/NINDS NIH HHS/United States
- P20RR15635/RR/NCRR NIH HHS/United States (VSports)
- P01 MH064570/MH/NIMH NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
- 2R37 NS36126/NS/NINDS NIH HHS/United States
LinkOut - more resources (VSports app下载)
Full Text Sources
Research Materials
Miscellaneous

