Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation
- PMID: 19109145
- PMCID: PMC2610348
- DOI: 10.4049/jimmunol.182.1.148
"V体育官网" Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation
Abstract
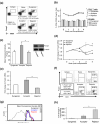
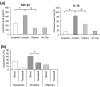
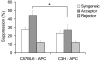
Foxp3 expressing CD4(+)CD25(+) regulatory T cells (Tregs) have been shown to prevent allograft rejection in clinical and animal models of transplantation. However, the role of Foxp3 in regulating Treg function, and the kinetics and mechanism of action of Tregs in inducing allograft tolerance in transplantation, are still not fully understood. Thus, we investigated the kinetics and function of Tregs in a mouse model of orthotopic corneal transplantation, the most common form of tissue grafting worldwide. In this study, using in vitro functional assays and in vivo Treg adoptive transfer assays, we show that far more relevant than Treg frequency is their level of Foxp3 expression, which is directly associated with the potential of Tregs to prevent allograft rejection by producing regulatory cytokines and suppressing effector T cell activation VSports手机版. In addition, our data clearly demonstrate that Tregs primarily suppress the induction of alloimmunity in regional draining lymph nodes rather than suppressing the effector phase of the immune response in the periphery. These findings provide new insights on Treg dynamics in transplantation, which are crucial for designing therapeutic strategies to modulate Treg function and to optimize Treg-based cell therapies for clinical translation. .
Figures
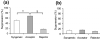
References
-
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003;3:199–210. - PubMed
-
- Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am. J. Transplant. 2007;7:1457–1463. - PubMed
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. - PubMed (V体育平台登录)
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
Publication types (VSports在线直播)
- "V体育官网入口" Actions
- Actions (V体育2025版)
MeSH terms
- VSports最新版本 - Actions
- VSports最新版本 - Actions
- Actions (VSports注册入口)
- "V体育安卓版" Actions
- "VSports app下载" Actions
- VSports app下载 - Actions
- "V体育ios版" Actions
- V体育平台登录 - Actions
- VSports手机版 - Actions
- "V体育官网入口" Actions
- "V体育2025版" Actions
- "V体育平台登录" Actions
- "V体育安卓版" Actions
Substances
- "V体育平台登录" Actions
- VSports手机版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

