"V体育ios版" Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition?
- PMID: 19075016
- PMCID: PMC2635052
- DOI: "V体育2025版" 10.1074/jbc.M807869200
Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition?
Abstract
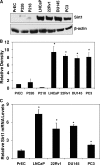
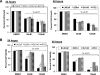
Prostate cancer (PCa) is a major age-related malignancy, and according to estimates from the American Cancer Society, a man's chance of developing this cancer significantly increases with increasing age, from 1 in 10,149 by age 39 to 1 in 38 by age 59 to 1 in 7 by age 70. Therefore, it is important to identify the causal connection between mechanisms of aging and PCa. Employing in vitro and in vivo approaches, in this study, we tested the hypothesis that SIRT1, which belongs to the Sir2 (silent information regulator 2) family of sirtuin class III histone deacetylases, is overexpressed in PCa, and its inhibition will have antiproliferative effects in human PCa cells. Our data demonstrated that SIRT1 was significantly overexpressed in human PCa cells (DU145, LNCaP, 22Rnu1, and PC3) compared with normal prostate epithelial cells (PrEC) at protein, mRNA, and enzymatic activity levels. SIRT1 was also found to be overexpressed in human PCa tissues compared with adjacent normal prostate tissue VSports手机版. Interestingly, our data demonstrated that SIRT1 inhibition via nicotinamide and sirtinol (at the activity level) as well as via short hairpin RNA-mediated RNA interference (at the genetic level) resulted in a significant inhibition in the growth and viability of human PCa cells while having no effect on normal prostate epithelial cells. Further, we found that inhibition of SIRT1 caused an increase in FOXO1 acetylation and transcriptional activation in PCa cells. Our data suggested that SIRT1, via inhibiting FOXO1 activation, could contribute to the development of PCa. We suggest that SIRT1 could serve as a target toward developing novel strategies for PCa management. .
Figures





References
-
- Longo, V. D., and Kennedy, B. K. (2006) Cell 126 257-268 - "V体育2025版" PubMed
-
- Marmorstein, R. (2004) Biochem. Soc. Trans. 32 904-909 - PubMed
-
- Michan, S., and Sinclair, D. (2007) Biochem. J. 404 1-13 - PMC (VSports最新版本) - PubMed
-
- Sauve, A. A., Wolberger, C., Schramm, V. L., and Boeke, J. D. (2006) Annu. Rev. Biochem. 75 435-465 - "V体育官网" PubMed
-
- Haigis, M. C., and Guarente, L. P. (2006) Genes Dev. 20 2913-2921 - VSports注册入口 - PubMed
"V体育ios版" Publication types
MeSH terms
- Actions (VSports app下载)
- V体育平台登录 - Actions
- V体育安卓版 - Actions
- Actions (VSports注册入口)
- "VSports注册入口" Actions
- VSports - Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
- Actions (V体育安卓版)
Substances
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- Actions (VSports app下载)
- V体育官网 - Actions
- "V体育官网" Actions
- "V体育安卓版" Actions
- "VSports app下载" Actions
- Actions (VSports最新版本)
- VSports注册入口 - Actions
Grants and funding
LinkOut - more resources
V体育官网 - Full Text Sources
VSports手机版 - Other Literature Sources
Medical
Research Materials
Miscellaneous

