Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin
- PMID: 19033648
- PMCID: "VSports注册入口" PMC2613454
- DOI: 10.1172/JCI36226
Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin (V体育安卓版)
"V体育安卓版" Abstract
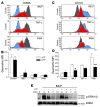
Apoptosis is a noninflammatory, programmed form of cell death. One mechanism underlying the non-phlogistic nature of the apoptosis program is the swift phagocytosis of the dying cells. How apoptotic cells attract mononuclear phagocytes and not granulocytes, the professional phagocytes that accumulate at sites of inflammation, has not been determined. Here, we show that apoptotic human cell lines of diverse lineages synthesize and secrete lactoferrin, a pleiotropic glycoprotein with known antiinflammatory properties. We further demonstrated that lactoferrin selectively inhibited migration of granulocytes but not mononuclear phagocytes, both in vitro and in vivo. Finally, we were able to attribute this antiinflammatory function of lactoferrin to its effects on granulocyte signaling pathways that regulate cell adhesion and motility. Together, our results identify lactoferrin as an antiinflammatory component of the apoptosis milieu and define what we believe to be a novel antiinflammatory property of lactoferrin: the ability to function as a negative regulator of granulocyte migration VSports手机版. .
Figures


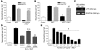
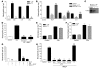



References
Publication types
MeSH terms
- VSports在线直播 - Actions
- Actions (V体育ios版)
- Actions (V体育安卓版)
- "V体育官网入口" Actions
- "VSports在线直播" Actions
Substances
- "VSports注册入口" Actions
Grants and funding
LinkOut - more resources
V体育官网 - Full Text Sources
Other Literature Sources
Molecular Biology Databases

