Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression
- PMID: 19017641
- PMCID: PMC2613630
- DOI: 10.1074/jbc.M807325200
"VSports最新版本" Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression
Abstract
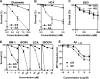
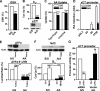
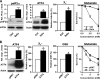
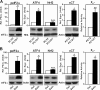
eIF2alpha is part of a multimeric complex that regulates cap-dependent translation. Phosphorylation of eIF2alpha (phospho-eIF2alpha) is induced by various forms of cell stress, resulting in changes to the proteome of the cell with two diametrically opposed consequences, adaptation to stress or initiation of programmed cell death. In contrast to the robust eIF2alpha phosphorylation seen in response to acute insults, less is known about the functional role of basal levels of eIF2alpha phosphorylation. Here we show that mouse embryonic fibroblasts expressing a nonphosphorylatable eIF2alpha have enhanced sensitivity to diverse toxic insults, including amyloid beta-(1-42) peptide (Abeta), a key factor in the pathogenesis of Alzheimer disease VSports手机版. This correlates with impaired glutathione metabolism because of down-regulation of the light chain, xCT, of the cystine/glutamate antiporter system X(-)(c). The mechanistic link between the absence of phospho-eIF2alpha and xCT expression is nuclear factor ATF4. Consistent with these findings, long term activation of the phospho-eIF2alpha/ATF4/xCT signaling module by the specific eIF2alpha phosphatase inhibitor, salubrinal, induces resistance against oxidative glutamate toxicity in the hippocampal cell line HT22 and primary cortical neurons. Furthermore, in PC12 cells selected for resistance against Abeta, increased activity of the phospho-eIF2alpha/ATF4/xCT module contributes to the resistant phenotype. In wild-type PC12 cells, activation of this module by salubrinal ameliorates the response to Abeta. Furthermore, in human brains, ATF4 and phospho-eIF2alpha levels are tightly correlated and up-regulated in Alzheimer disease, most probably representing an adaptive response against disease-related cellular stress rather than a correlate of neurodegeneration. .
Figures


References (V体育官网入口)
Publication types
- VSports app下载 - Actions
MeSH terms
- Actions (VSports手机版)
- V体育安卓版 - Actions
- Actions (V体育官网入口)
- "V体育ios版" Actions
- Actions (V体育官网)
- Actions (VSports手机版)
- "V体育2025版" Actions
Substances
- Actions (VSports最新版本)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

