Regulated expression of a tumor-associated antigen reveals multiple levels of T-cell tolerance in a mouse model of lung cancer
- PMID: 19010921
- PMCID: PMC2597052
- DOI: 10.1158/0008-5472.CAN-08-2634
Regulated expression of a tumor-associated antigen reveals multiple levels of T-cell tolerance in a mouse model of lung cancer
Abstract
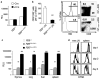
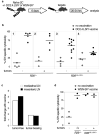
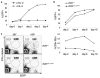
Maximizing the potential of cancer immunotherapy requires model systems that closely recapitulate human disease to study T-cell responses to tumor antigens and to test immunotherapeutic strategies. We have created a new system that is compatible with Cre-LoxP-regulatable mouse cancer models in which the SIY antigen is specifically overexpressed in tumors, mimicking clinically relevant TAAs. To show the utility of this system, we have characterized SIY-reactive T cells in the context of lung adenocarcinoma, revealing multiple levels of antigen-specific T-cell tolerance that serve to limit an effective antitumor response. Thymic deletion reduced the number of SIY-reactive T cells present in the animals. When potentially self-reactive T cells in the periphery were activated, they were efficiently eliminated. Inhibition of apoptosis resulted in more persistent self-reactive T cells, but these cells became anergic to antigen stimulation VSports手机版. Finally, in the presence of tumors overexpressing SIY, SIY-specific T cells required a higher level of costimulation to achieve functional activation. This system represents a valuable tool in which to explore sources contributing to T-cell tolerance of cancer and to test therapies aimed at overcoming this tolerance. .
Figures



References
-
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. - V体育官网 - PubMed
-
- Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178(3):1268–76. - PubMed
-
- Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–49. - "V体育2025版" PMC - PubMed
Publication types
- "V体育官网入口" Actions
MeSH terms (VSports注册入口)
- Actions (VSports注册入口)
- "VSports在线直播" Actions
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- V体育2025版 - Actions
- "VSports手机版" Actions
Substances (VSports)
- "V体育2025版" Actions
Grants and funding
"VSports" LinkOut - more resources
Full Text Sources (VSports在线直播)
Other Literature Sources
"V体育官网入口" Medical
Molecular Biology Databases

