HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition
- PMID: 18832382
- PMCID: PMC2662269
- DOI: 10.1074/jbc.M802016200
HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition (VSports)
Abstract
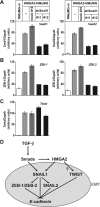
Epithelial-mesenchymal transition (EMT) is important during embryonic cell layer movement and tumor cell invasiveness. EMT converts adherent epithelial cells to motile mesenchymal cells, favoring metastasis in the context of cancer progression. Transforming growth factor-beta (TGF-beta) triggers EMT via intracellular Smad transducers and other signaling proteins. We previously reported that the high mobility group A2 (HMGA2) gene is required for TGF-beta to elicit EMT in mammary epithelial cells. In the present study we investigated the molecular mechanisms by which HMGA2 induces EMT VSports手机版. We found that HMGA2 regulates expression of many important repressors of E-cadherin. Among these, we analyzed in detail the zinc-finger transcription factor SNAIL1, which plays key roles in tumor progression and EMT. We demonstrate that HMGA2 directly binds to the SNAIL1 promoter and acts as a transcriptional regulator of SNAIL1 expression. Furthermore, we observed that HMGA2 cooperates with the TGF-beta/Smad pathway in regulating SNAIL1 gene expression. The mechanism behind this cooperation involves physical interaction between these factors, leading to an increased binding of Smads to the SNAIL1 promoter. SNAIL1 seems to play the role of a master effector downstream of HMGA2 for induction of EMT, as SNAIL1 knock-down partially reverts HMGA2-induced loss of epithelial differentiation. The data propose that HMGA2 acts in a gene-specific manner to orchestrate the transcriptional network necessary for the EMT program. .
Figures (V体育安卓版)



References
-
- Thiery, J.-P., and Sleeman, J. P. (2006) Nat. Rev. Mol. Cell Biol. 7 131-142 - "V体育ios版" PubMed
-
- Peinado, H., Portillo, F., and Cano, A. (2004) Int. J. Dev. Biol. 48 365-375 - PubMed
-
- Peinado, H., Olmeda, D., and Cano, A. (2007) Nat. Rev. Cancer 7 415-428 - VSports最新版本 - PubMed
-
- Yang, J., Mani, S. A., Donaher, J. L., Ramaswamy, S., Itzykson, R. A., Come, C., Savagner, P., Gitelman, I., Richardson, A., and Weinberg, R. A. (2004) Cell 117 927-939 - PubMed
-
- Kondo, M., Cubillo, E., Tobiume, K., Shirakihara, T., Fukuda, N., Suzuki, H., Shimizu, K., Takehara, K., Cano, A., Saitoh, M., and Miyazono, K. (2004) Cell Death Differ. 11 1092-1101 - VSports最新版本 - PubMed
Publication types
- "V体育ios版" Actions
V体育2025版 - MeSH terms
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- "V体育官网入口" Actions
- Actions (V体育平台登录)
- "V体育官网" Actions
- "VSports app下载" Actions
- Actions (VSports手机版)
- "V体育官网" Actions
- "VSports手机版" Actions
- VSports手机版 - Actions
- "VSports注册入口" Actions
- V体育ios版 - Actions
Substances
- V体育官网入口 - Actions
- VSports - Actions
- VSports手机版 - Actions
- VSports在线直播 - Actions
LinkOut - more resources
Full Text Sources
"VSports在线直播" Molecular Biology Databases
Research Materials

