An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice
- PMID: 18724376
- PMCID: PMC3446847
- DOI: 10.1038/nm.1865
An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice
Abstract
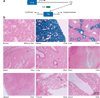
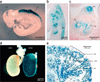
We describe a transgenic mouse line, Pax8-rtTA, which, under control of the mouse Pax8 promoter, directs high levels of expression of the reverse tetracycline-dependent transactivator (rtTA) to all proximal and distal tubules and the entire collecting duct system of both embryonic and adult kidneys. Using crosses of Pax8-rtTA mice with tetracycline-responsive c-MYC mice, we established a new, inducible model of polycystic kidney disease that can mimic adult onset and that shows progression to renal malignant disease. When targeting the expression of transforming growth factor beta-1 to the kidney, we avoided early lethality by discontinuous treatment and successfully established an inducible model of renal fibrosis. Finally, a conditional knockout of the gene encoding tuberous sclerosis complex-1 was achieved, which resulted in the early outgrowth of giant polycystic kidneys reminiscent of autosomal recessive polycystic kidney disease. These experiments establish Pax8-rtTA mice as a powerful tool for modeling renal diseases in transgenic mice VSports手机版. .
Figures



VSports最新版本 - References
-
- Gossen M, et al. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. - PubMed
-
- Kistner A, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. USA. 1996;93:10933–10938. - V体育安卓版 - PMC - PubMed
-
- Poleev A, et al. PAX8, a human paired box gene: isolation and expression in developing thyroid, kidney and Wilms’ tumors. Development. 1992;116:611–623. - VSports注册入口 - PubMed
-
- Plachov D, et al. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–651. - PubMed (V体育官网入口)
Publication types
- "V体育官网" Actions
MeSH terms
- "V体育ios版" Actions
- "V体育平台登录" Actions
- "V体育ios版" Actions
- Actions (VSports app下载)
- V体育2025版 - Actions
- "V体育ios版" Actions
- Actions (V体育官网)
- Actions (V体育官网入口)
- "VSports在线直播" Actions
- "VSports在线直播" Actions
- Actions (V体育ios版)
Substances
- Actions (VSports在线直播)
- "VSports在线直播" Actions
- Actions (V体育官网入口)
- "V体育平台登录" Actions
- Actions (VSports注册入口)
- "V体育ios版" Actions
- "VSports" Actions
- Actions (V体育官网入口)
"V体育安卓版" Grants and funding
VSports注册入口 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

