Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity
- PMID: 18715135
- PMCID: PMC2689082
- DOI: 10.1359/jbmr.080817
Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity (VSports注册入口)
Abstract
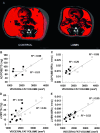
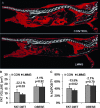
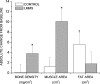
Mesenchymal stem cells (MSCs) are defined by their ability to self-renew and differentiate into the cells that form mesodermal tissues such as bone and fat. Low magnitude mechanical signals (LMMS) have been shown to be anabolic to bone and have been recently reported to suppress the development of fat in normal animals fed a regular diet. Using male C57BL/6J mice, the ability of LMMS (0. 2g, 90-Hz signal applied for 15 min/d, 5 d/wk) to simultaneously promote bone formation and prevent diet-induced obesity was correlated to mechanical influences on the molecular environment of the bone marrow, as indicated by the population dynamics and lineage commitment of MSCs VSports手机版. Six weeks of LMMS increased the overall marrow-based stem cell population by 37% and the number of MSCs by 46%. Concomitant with the increase in stem cell number, the differentiation potential of MSCs in the bone marrow was biased toward osteoblastic and against adipogenic differentiation, as reflected by upregulation of the transcription factor Runx2 by 72% and downregulation of PPARgamma by 27%. The phenotypic impact of LMMS on MSC lineage determination was evident at 14 wk, where visceral adipose tissue formation was suppressed by 28%, whereas trabecular bone volume fraction in the tibia was increased by 11%. Translating this to the clinic, a 1-yr trial in young women (15-20 yr; n = 48) with osteopenia showed that LMMS increased trabecular bone in the spine and kept visceral fat at baseline levels, whereas control subjects showed no change in BMD, yet an increase in visceral fat. Mechanical modulation of stem cell proliferation and differentiation indicates a unique therapeutic target to aid in tissue regeneration and repair and may represent the basis of a nonpharmacologic strategy to simultaneously prevent obesity and osteoporosis. .
Figures

References
-
- Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–45977. - PubMed
-
- David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. - PubMed (VSports注册入口)
-
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131:242–256. - V体育平台登录 - PubMed
-
- Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: Implications for tissue engineering. Adv Drug Deliv Rev. 2007;59:1309–1318. - V体育官网 - PubMed
"VSports最新版本" Publication types
- "V体育平台登录" Actions
MeSH terms
- Actions (V体育2025版)
- "VSports最新版本" Actions
- Actions (V体育ios版)
- Actions (V体育平台登录)
- V体育平台登录 - Actions
- VSports注册入口 - Actions
- Actions (V体育安卓版)
- VSports在线直播 - Actions
- V体育2025版 - Actions
Grants and funding
VSports - LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

