PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome
- PMID: 18522850
- PMCID: V体育ios版 - PMC2892934
- DOI: VSports在线直播 - 10.1016/j.stem.2008.03.009
PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome
Abstract
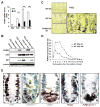
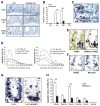
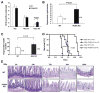
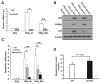
Radiation is one of the most effective cancer treatments. However, gastrointestinal (GI) syndrome is a major limiting factor in abdominal and pelvic radiotherapy. The loss of crypt stem cells or villus endothelial cells has been suggested to be responsible for radiation-induced intestinal damage. We report here a critical role of the BH3-only protein p53 upregulated modulator of apoptosis (PUMA) in the radiosensitivity of intestinal epithelium and pathogenesis of GI syndrome. PUMA was induced in a p53-dependent manner and mediated radiation-induced apoptosis via the mitochondrial pathway in the intestinal mucosa VSports手机版. PUMA-deficient mice exhibited blocked apoptosis in the intestinal progenitor and stem cells, enhanced crypt proliferation and regeneration, and prolonged survival following lethal doses of radiation. Unexpectedly, PUMA deficiency had little effect on radiation-induced intestinal endothelial apoptosis. Suppressing PUMA expression by antisense oligonucleotides provided significant intestinal radioprotection. Therefore, PUMA-mediated apoptosis in the progenitor and stem cell compartments is crucial for radiation-induced intestinal damage. .
Figures


VSports app下载 - Comment in
-
VSports app下载 - Puma: mauling the intestinal crypt.Cell Stem Cell. 2008 Jun 5;2(6):517-8. doi: 10.1016/j.stem.2008.05.014. Cell Stem Cell. 2008. PMID: 18522842
References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Booth D, Potten CS. Protection against mucosal injury by growth factors and cytokines. J Natl Cancer Inst Monogr. 2001;29:16–20. - PubMed
-
- Carson-Walter EB, Hampton J, Shue E, Geynisman DM, Pillai PK, Sathanoori R, Madden SL, Hamilton RL, Walter KA. Plasma-lemmal vesicle associated protein-1 is a novel marker implicated in brain tumor angiogenesis. Clin Cancer Res. 2005;11:7643–7650. - VSports注册入口 - PubMed
-
- Ch’ang HJ, Maj JG, Paris F, Xing HR, Zhang J, Truman JP, Cardon-Cardo C, Haimovitz-Friedman A, Kolesnick R, Fuks Z. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 2005;11:484–490. - PubMed (V体育安卓版)
Publication types
MeSH terms
- Actions (VSports手机版)
- V体育ios版 - Actions
- "V体育2025版" Actions
- "V体育官网" Actions
- VSports app下载 - Actions
- "VSports" Actions
- Actions (VSports最新版本)
- VSports在线直播 - Actions
- "VSports" Actions
- Actions (VSports最新版本)
- Actions (VSports注册入口)
Substances
- Actions (V体育官网入口)
- "V体育官网" Actions
- VSports最新版本 - Actions
- VSports最新版本 - Actions
Grants and funding
"V体育ios版" LinkOut - more resources
Full Text Sources
Molecular Biology Databases
VSports在线直播 - Research Materials
Miscellaneous

