V体育官网 - An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments
- PMID: 18483268
- PMCID: PMC2587151
- DOI: "VSports最新版本" 10.1158/0008-5472.CAN-08-0215
An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments
Abstract
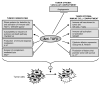
Overexpression of transforming growth factor beta (TGF-beta) is frequently associated with metastasis and poor prognosis, and TGF-beta antagonism has been shown to prevent metastasis in preclinical models with surprisingly little toxicity. Here, we have used the transplantable 4T1 model of metastatic breast cancer to address underlying mechanisms VSports手机版. We showed that efficacy of the anti-TGF-beta antibody 1D11 in suppressing metastasis was dependent on a synergistic combination of effects on both the tumor parenchyma and microenvironment. The main outcome was a highly significant enhancement of the CD8+ T-cell-mediated antitumor immune response, but effects on the innate immune response and on angiogenesis also contributed to efficacy. Treatment with 1D11 increased infiltration of natural killer cells and T cells at the metastatic site, and enhanced expression of coactivators (NKG2D) and cytotoxic effectors (perforin and granzyme B) on CD8+ T cells. On the tumor cells, increased expression of an NKG2D ligand (Rae1gamma) and of a death receptor (TNFRSF1A) contributed to enhanced immune cell-mediated recognition and lysis. The data suggest that elevated TGF-beta expression in the tumor microenvironment modulates a complex web of intercellular interactions that aggregately promote metastasis and progression. TGF-beta antibodies reverse this effect, and the absence of a major effect of TGF-beta antagonism on any one cell compartment may be critical for a good therapeutic window and the avoidance of autoimmune complications. .
Figures
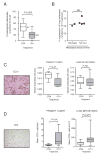
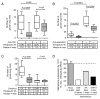
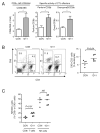

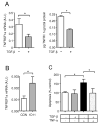
References
-
- Pardali K, Moustakas A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. - PubMed
-
- Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. - PubMed
-
- Gold LI. The role for transforming growth factor-β (TGF-β) in human cancer. Crit Rev Oncog. 1999;10:303–60. - PubMed
-
- Teicher BA. Transforming Growth Factor-β and the Immune Response to Malignant Disease. Clin Cancer Res. 2007;13:6247–51. - "VSports最新版本" PubMed
-
- Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–20. - PubMed
Publication types
- V体育平台登录 - Actions
- "V体育ios版" Actions
MeSH terms
- "V体育安卓版" Actions
- V体育ios版 - Actions
- V体育2025版 - Actions
- "V体育ios版" Actions
- "VSports手机版" Actions
Substances
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources
Full Text Sources (V体育平台登录)
V体育官网入口 - Other Literature Sources
Research Materials

