Tumor-specific Th17-polarized cells eradicate large established melanoma
- PMID: 18354038
- PMCID: PMC2442746
- DOI: 10.1182/blood-2007-11-120998
Tumor-specific Th17-polarized cells eradicate large established melanoma
"V体育官网" Abstract
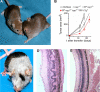
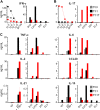
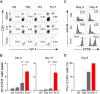
CD4+ T cells can differentiate into multiple effector subsets, but the potential roles of these subsets in anti-tumor immunity have not been fully explored. Seeking to study the impact of CD4+ T cell polarization on tumor rejection in a model mimicking human disease, we generated a new MHC class II-restricted, T-cell receptor (TCR) transgenic mouse model in which CD4+ T cells recognize a novel epitope in tyrosinase-related protein 1 (TRP-1), an antigen expressed by normal melanocytes and B16 murine melanoma VSports手机版. Cells could be robustly polarized into Th0, Th1, and Th17 subtypes in vitro, as evidenced by cytokine, chemokine, and adhesion molecule profiles and by surface markers, suggesting the potential for differential effector function in vivo. Contrary to the current view that Th1 cells are most important in tumor rejection, we found that Th17-polarized cells better mediated destruction of advanced B16 melanoma. Their therapeutic effect was critically dependent on interferon-gamma (IFN-gamma) production, whereas depletion of interleukin (IL)-17A and IL-23 had little impact. Taken together, these data indicate that the appropriate in vitro polarization of effector CD4+ T cells is decisive for successful tumor eradication. This principle should be considered in designing clinical trials involving adoptive transfer-based immunotherapy of human malignancies. .
Figures


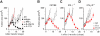
Comment in
-
Th17 and cancer: friends or foes?Blood. 2008 Jul 15;112(2):214. doi: 10.1182/blood-2008-04-149260. Blood. 2008. PMID: 18606882 No abstract available.
References
-
- Gerloni M, Zanetti M. CD4 T cells in tumor immunity. Springer Semin Immunopathol. 2005;27:37–48. - PubMed
-
- Jiang YZ, Barrett J. The allogeneic CD4+ T-cell-mediated graft-versus-leukemia effect. Leuk Lymphoma. 1997;28:33–42. - PubMed
-
- Sprangers B, Van Wijmeersch B, Fevery S, Waer M, Billiau AD. Experimental and clinical approaches for optimization of the graft-versus-leukemia effect. Nat Clin Pract Oncol. 2007;4:404–414. - PubMed
MeSH terms
- Actions (VSports app下载)
- Actions (V体育安卓版)
- "V体育ios版" Actions
- Actions (V体育平台登录)
Substances
- "V体育官网" Actions
- "VSports在线直播" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases (VSports在线直播)
Research Materials

